Summary information and primary citation
- PDB-id
- 8yli; DSSR-derived features in text and JSON formats
- Class
- transcription
- Method
- X-ray (2.9 Å)
- Summary
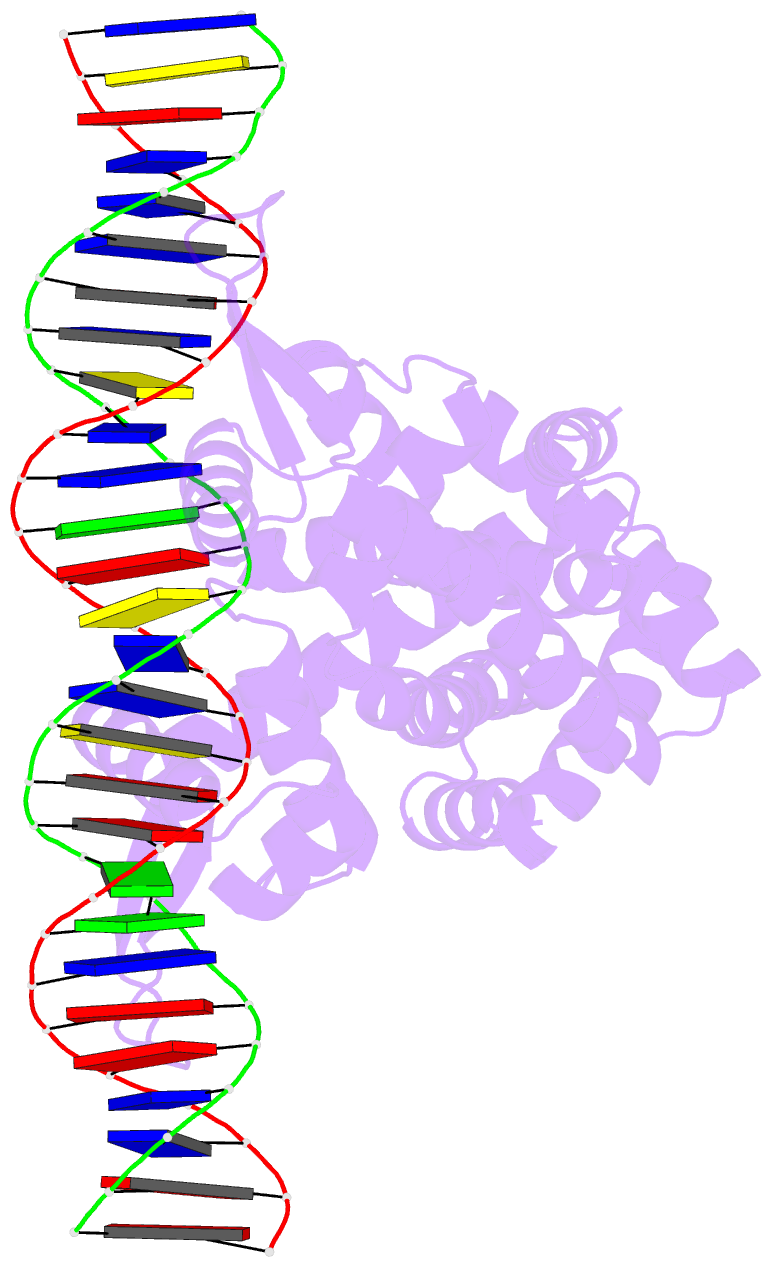
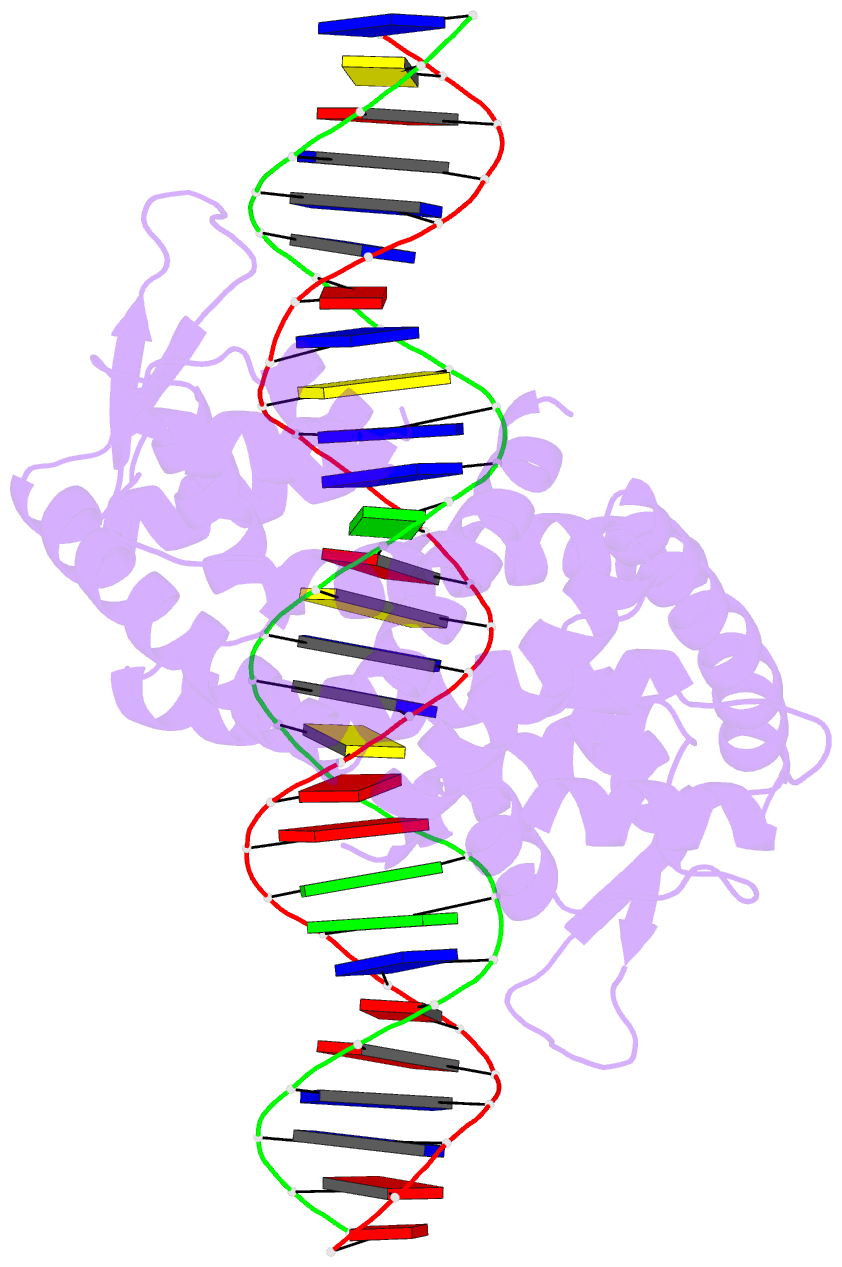
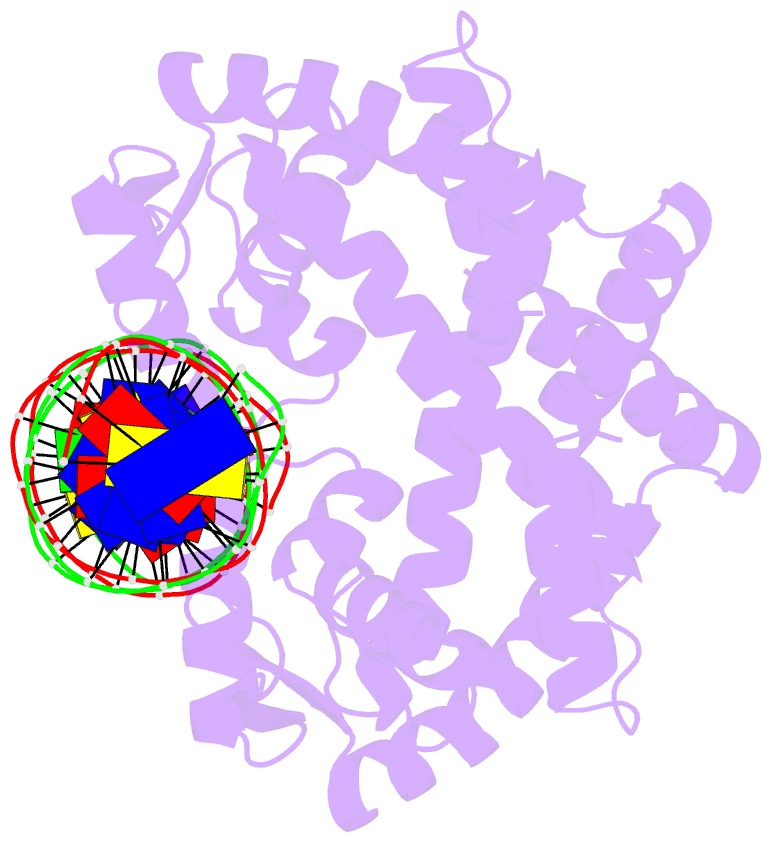
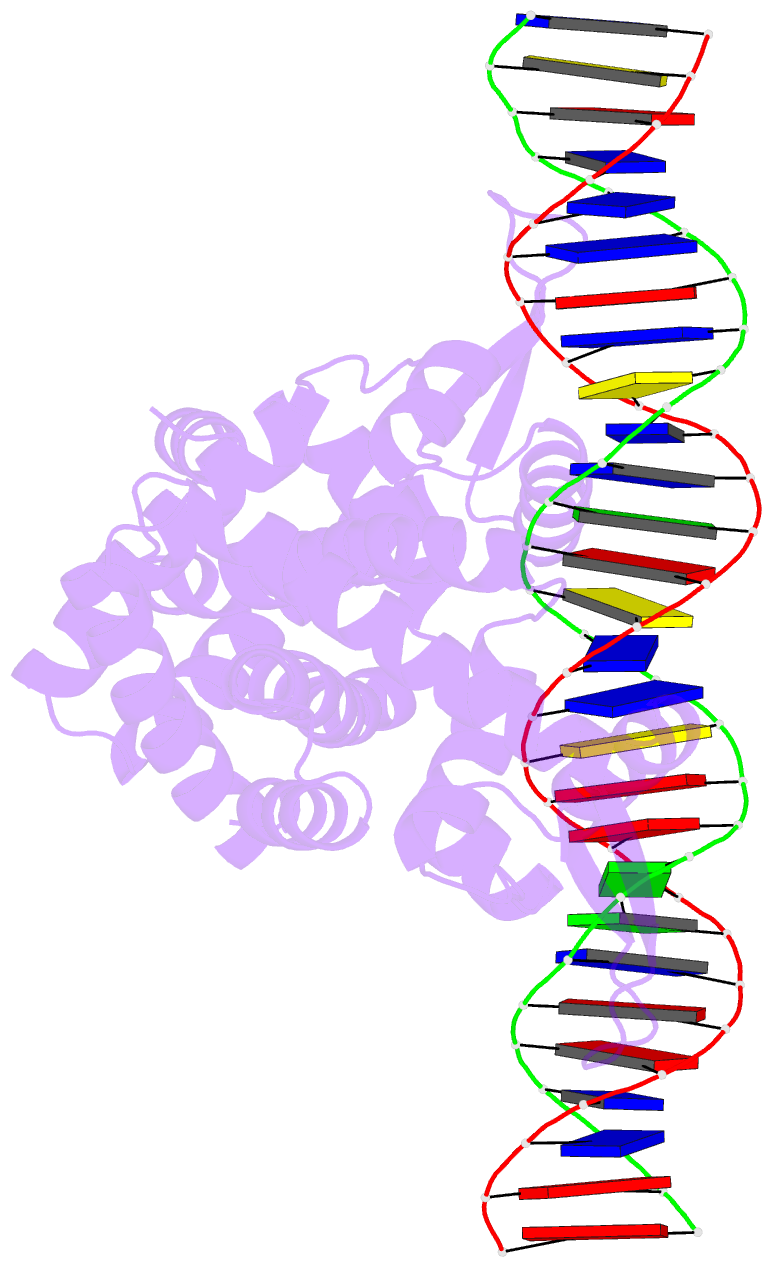
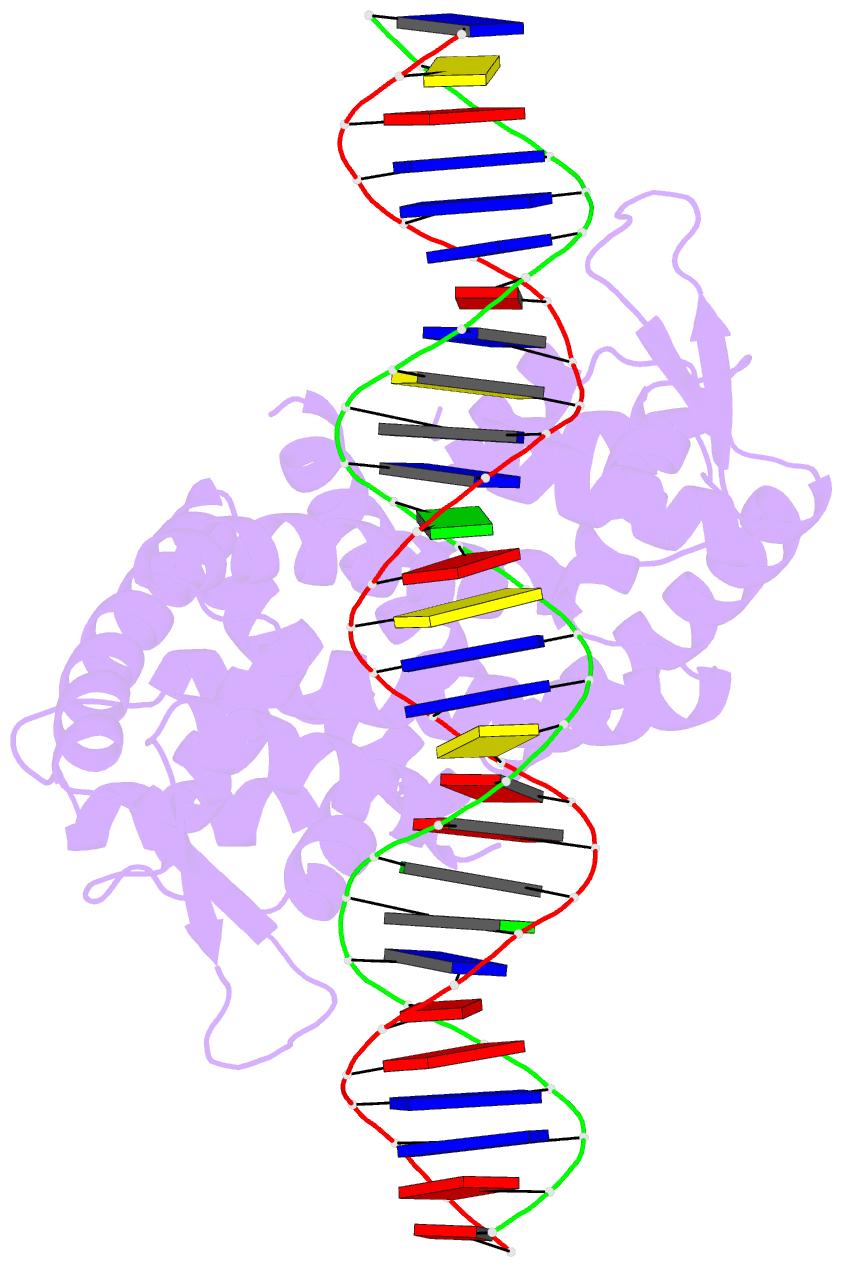
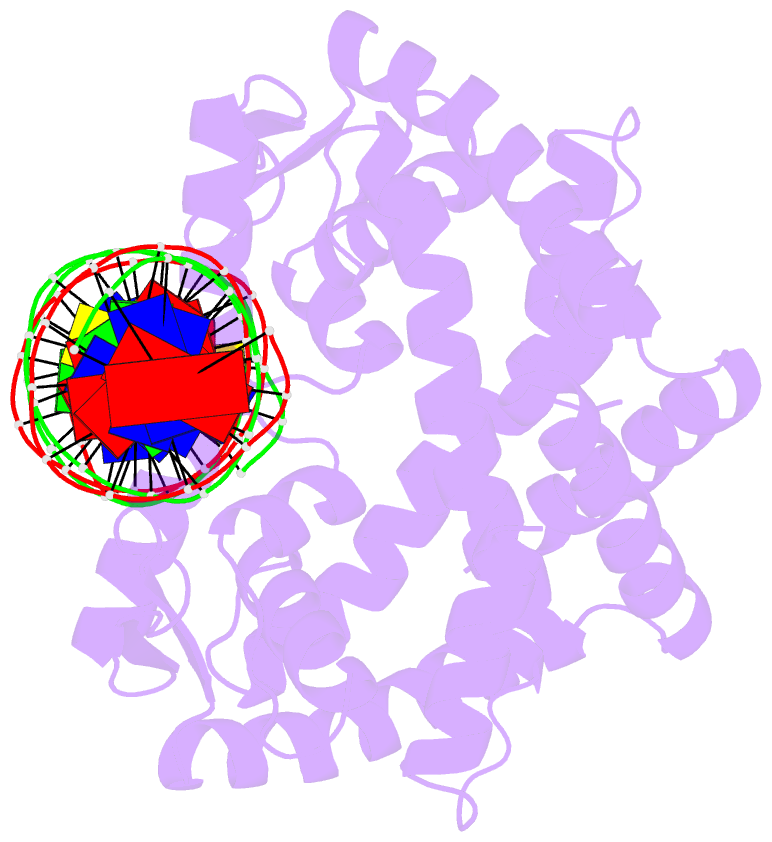
- Crystal structure of pectobacterium atrosepticum pecs in complex with operator DNA
- Reference
- Song WS, Ki DU, Cho HY, Kwon OH, Cho H, Yoon SI (2024): "Structural basis of transcriptional regulation by UrtR in response to uric acid." Nucleic Acids Res. doi: 10.1093/nar/gkae922.
- Abstract
- Uric acid (UA)-responsive transcriptional regulators (UrtRs), which belong to the multiple antibiotic resistance regulator (MarR) superfamily, transcriptionally coordinate virulence and metabolism in bacteria by modulating interactions with operator DNA in response to UA. To elucidate the transcriptional regulatory mechanism of UrtR, we structurally analyzed UrtR proteins, including PecS, MftR, and HucR, alone and in complex with UA or DNA. UrtR contains a dimerization domain (DD) and a winged helix-turn-helix domain (wHTHD) and forms a homodimer primarily via the DD, as observed for other MarR superfamily proteins. However, UrtRs are characterized by a unique N-terminal α-helix, which contributes to dimerization and UA recognition. In the absence of UA, the UrtR dimer symmetrically binds to the operator double-stranded DNA (dsDNA) by inserting its α4 recognition helix and β-stranded wing within the wHTHD into the major and minor grooves of dsDNA, respectively. Upon exposure to UA, UrtR accommodates UA in the intersubunit pocket between the DD and wHTHD. UA binding induces a conformational change in the major groove-binding core element of the UrtR wHTHD, generating a DNA binding-incompatible structure. This local allosteric mechanism of UrtR completely differs from that generally observed in other MarR superfamily members, in which the entire wHTHD undergoes effector-responsive global shifts.