Summary information and primary citation
- PDB-id
- 8flj; DSSR-derived features in text and JSON formats
- Class
- recombination, DNA binding protein, hydrolase-DNA
- Method
- cryo-EM (3.48 Å)
- Summary
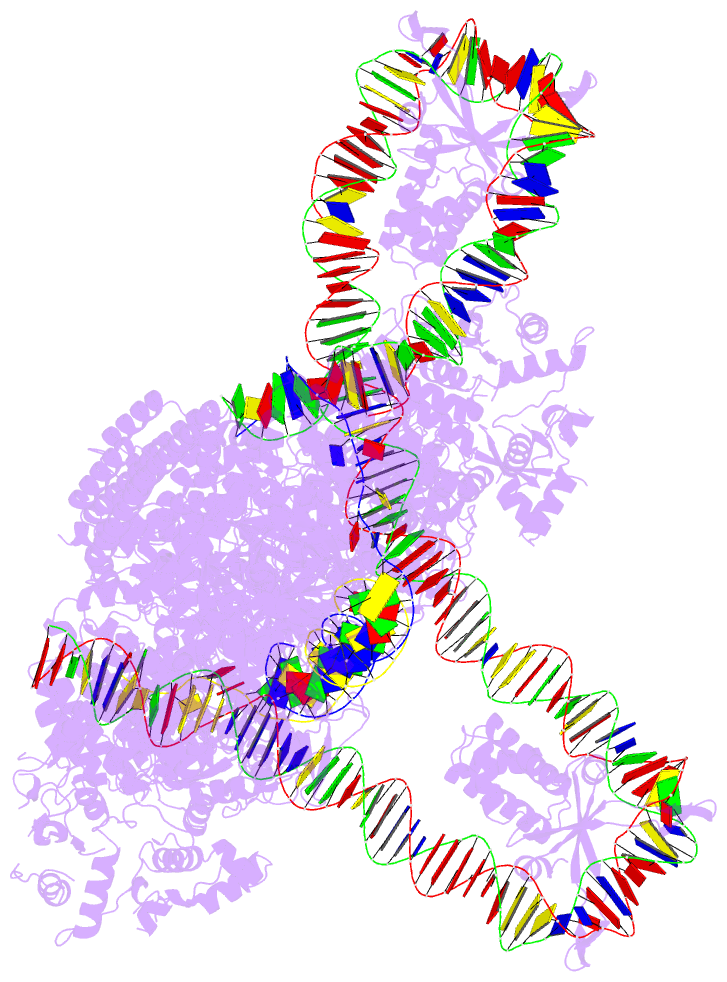
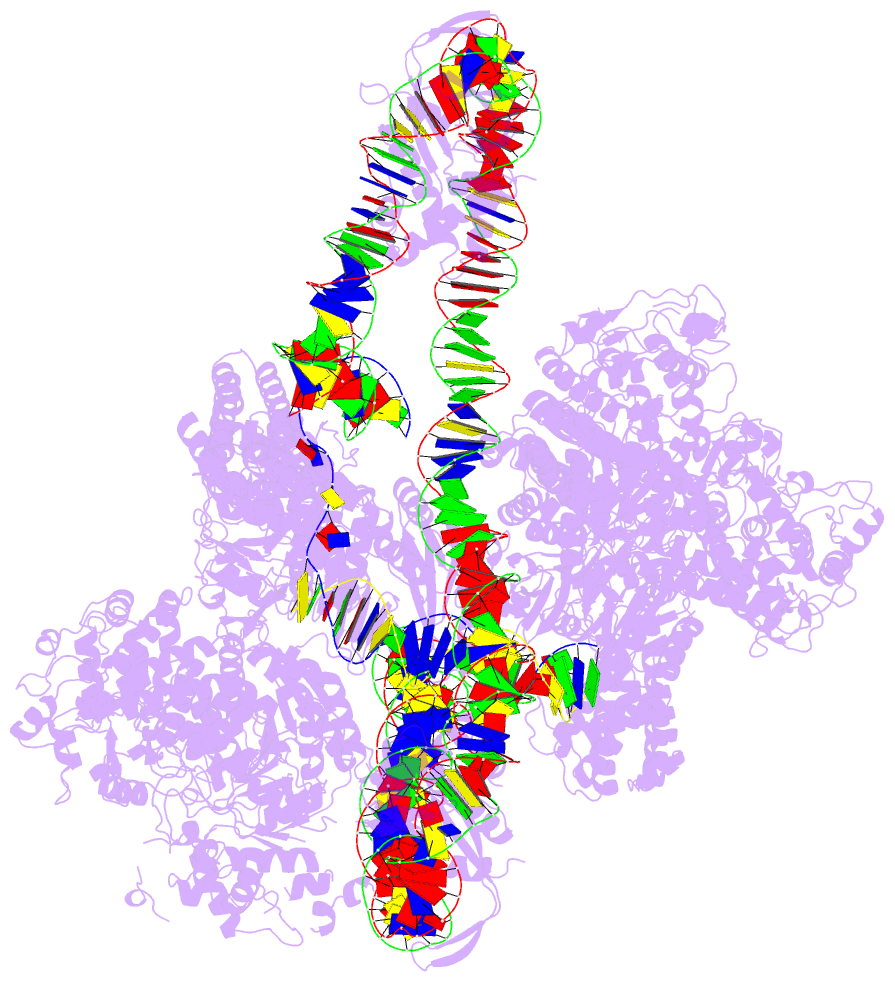
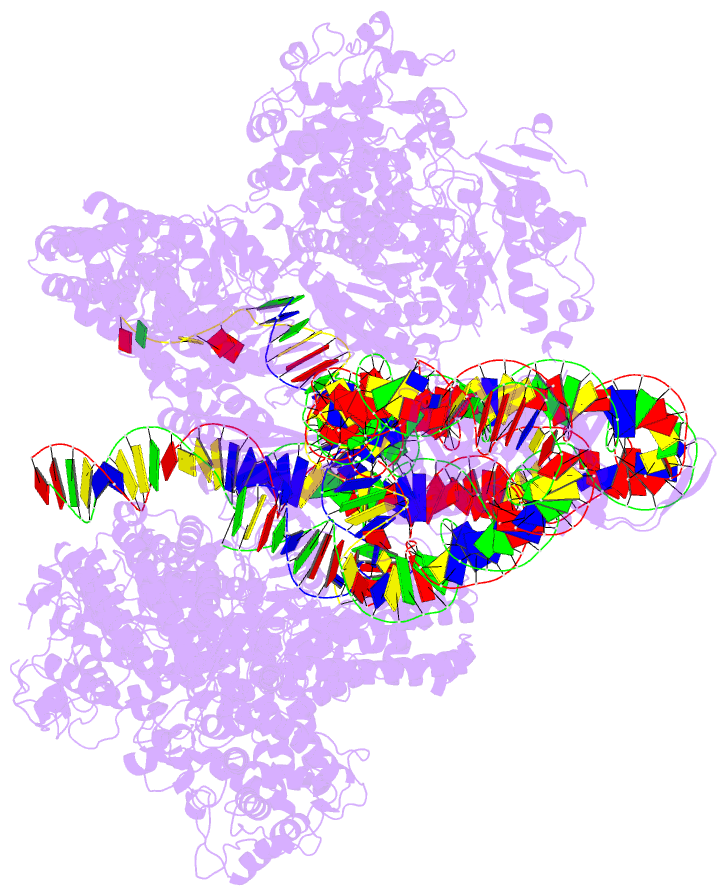
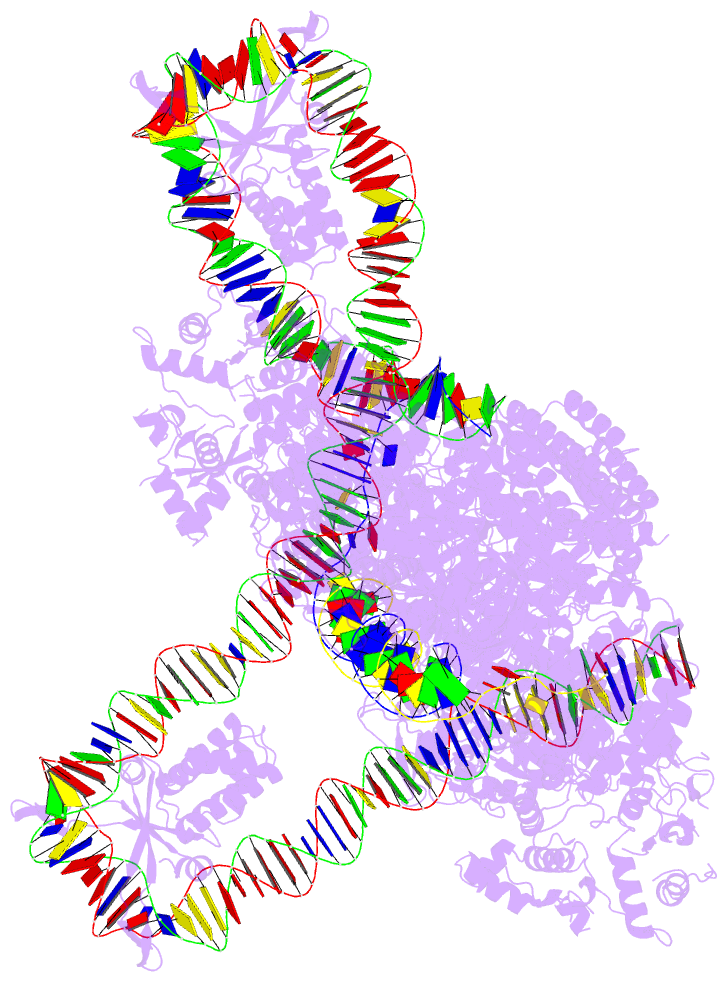
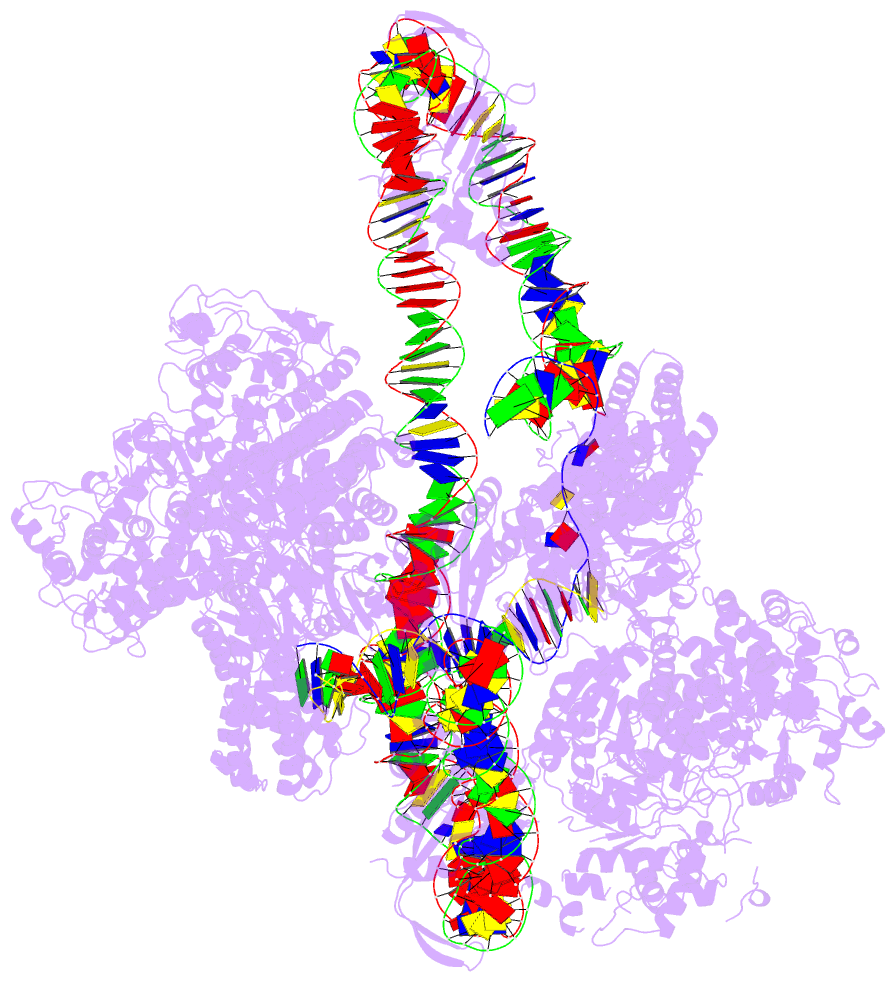
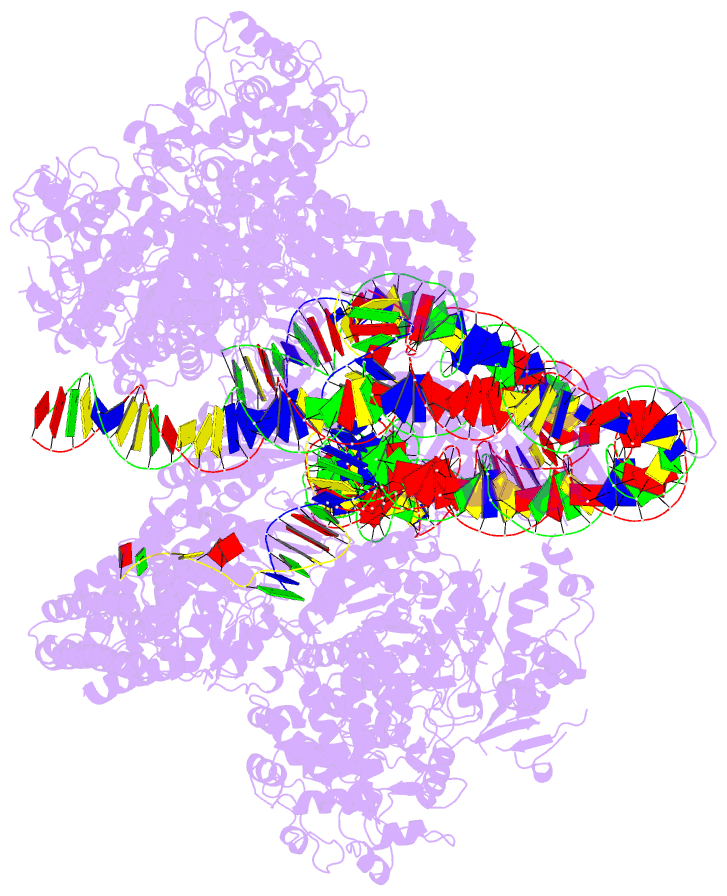
- Cas1-cas2-3 integrase and ihf bound to crispr leader, repeat and foreign DNA
- Reference
- Santiago-Frangos A, Henriques WS, Wiegand T, Gauvin CC, Buyukyoruk M, Graham AB, Wilkinson RA, Triem L, Neselu K, Eng ET, Lander GC, Wiedenheft B (2023): "Structure reveals why genome folding is necessary for site-specific integration of foreign DNA into CRISPR arrays." Nat.Struct.Mol.Biol., 30, 1675-1685. doi: 10.1038/s41594-023-01097-2.
- Abstract
- Bacteria and archaea acquire resistance to viruses and plasmids by integrating fragments of foreign DNA into the first repeat of a CRISPR array. However, the mechanism of site-specific integration remains poorly understood. Here, we determine a 560-kDa integration complex structure that explains how Pseudomonas aeruginosa Cas (Cas1-Cas2/3) and non-Cas proteins (for example, integration host factor) fold 150 base pairs of host DNA into a U-shaped bend and a loop that protrude from Cas1-2/3 at right angles. The U-shaped bend traps foreign DNA on one face of the Cas1-2/3 integrase, while the loop places the first CRISPR repeat in the Cas1 active site. Both Cas3 proteins rotate 100 degrees to expose DNA-binding sites on either side of the Cas2 homodimer, which each bind an inverted repeat motif in the leader. Leader sequence motifs direct Cas1-2/3-mediated integration to diverse repeat sequences that have a 5'-GT. Collectively, this work reveals new DNA-binding surfaces on Cas2 that are critical for DNA folding and site-specific delivery of foreign DNA.