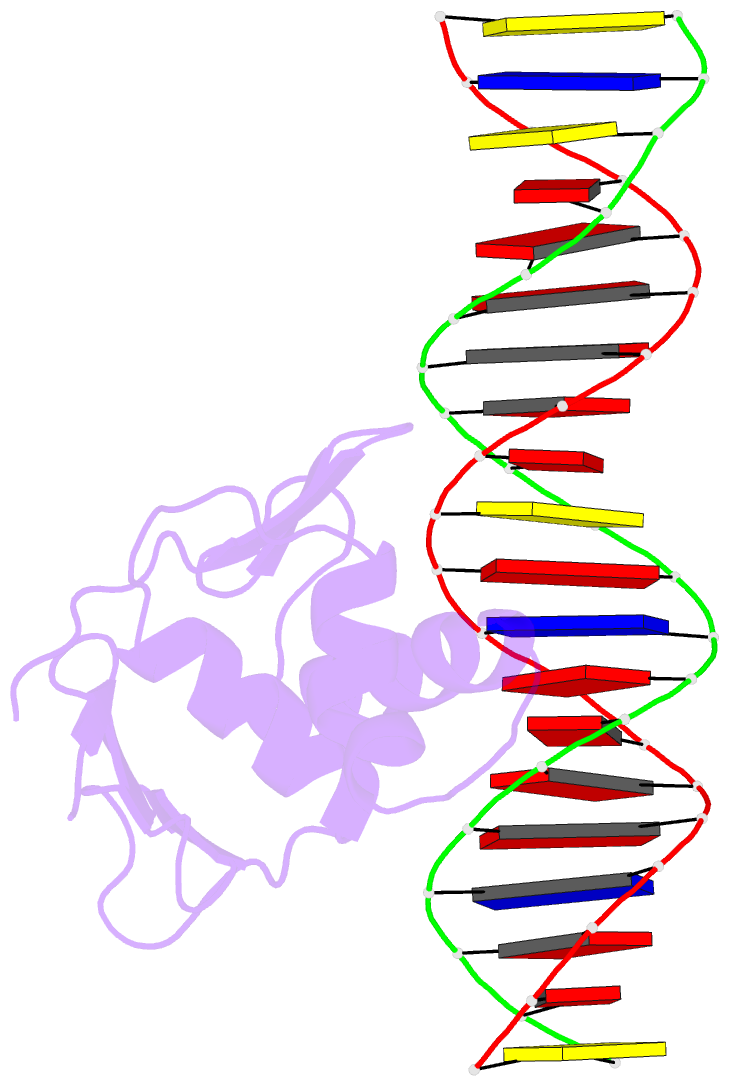
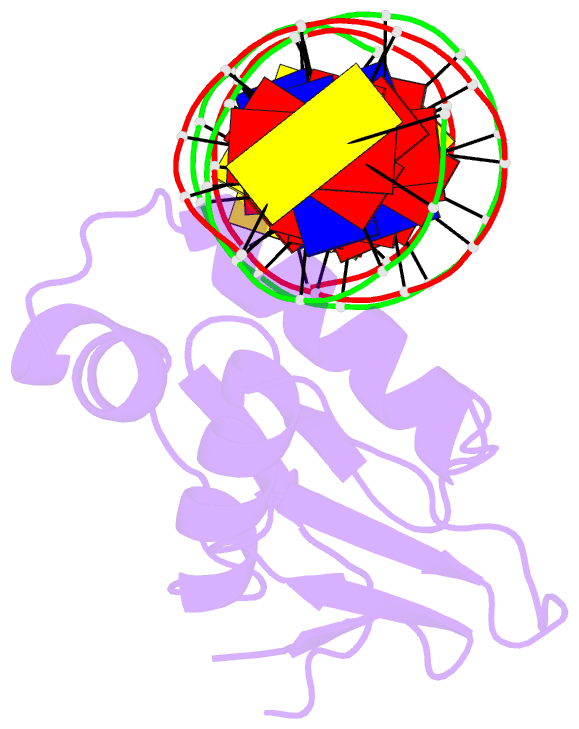
Summary information and primary citation
- PDB-id
-
8b4c;
SNAP-derived features in text and
JSON formats
- Class
- DNA binding protein
- Method
- X-ray (2.07 Å)
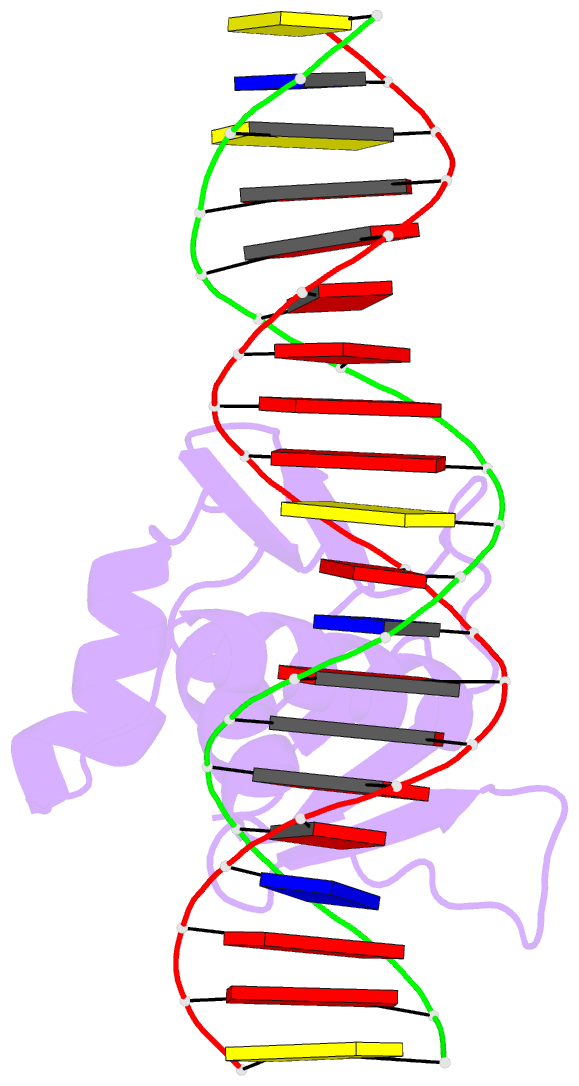
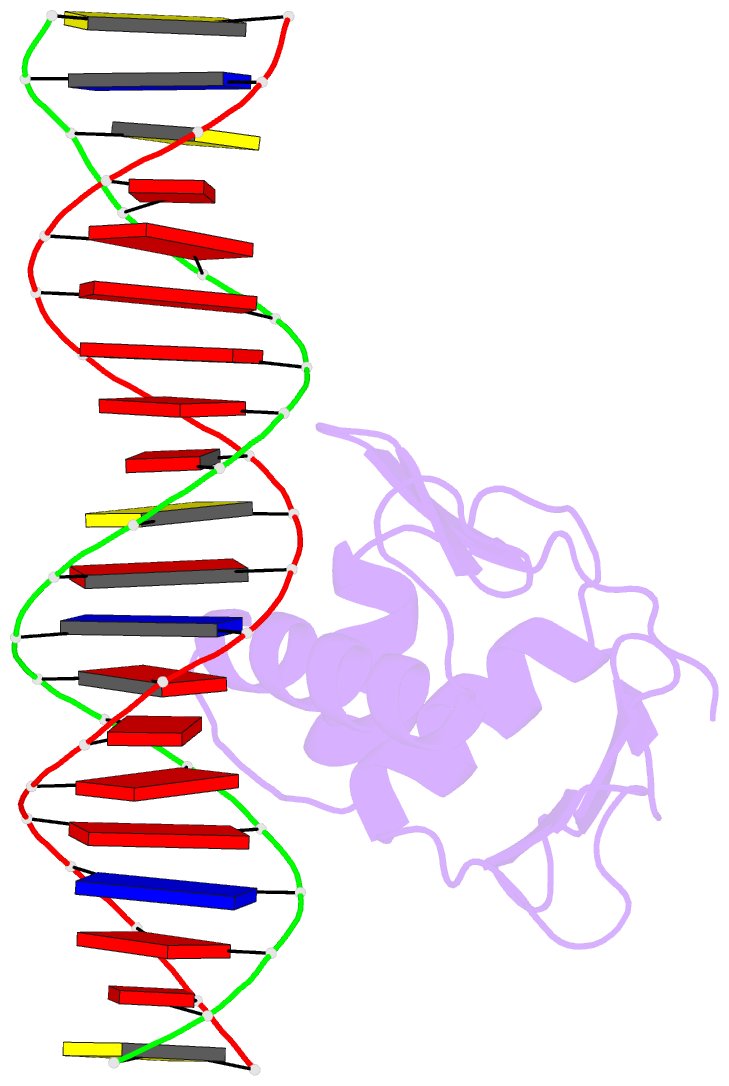
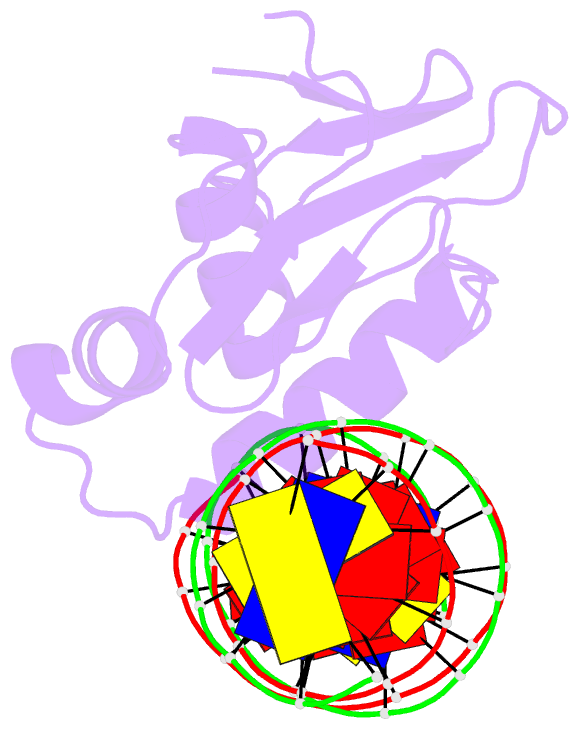
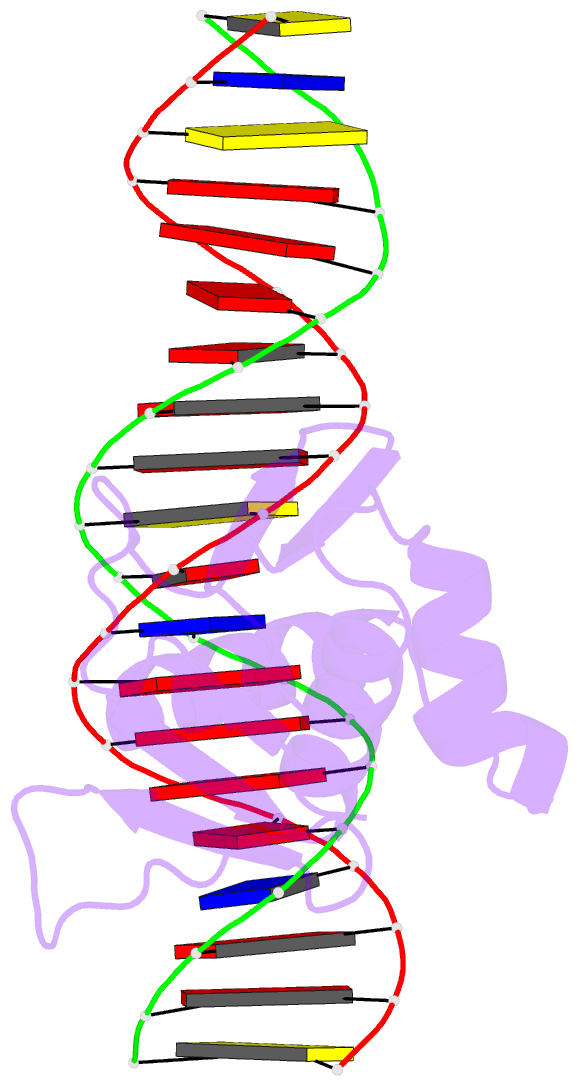
- Summary
- Toxr bacterial transcriptional regulator bound to 20 bp
toxt promoter DNA
- Reference
-
Canals A, Pieretti S, Muriel-Masanes M, El Yaman N,
Plecha SC, Thomson JJ, Fabrega-Ferrer M, Perez-Luque R,
Krukonis ES, Coll M (2023): "ToxR
activates the Vibrio cholerae virulence genes by
tethering DNA to the membrane through versatile binding
to multiple sites." Proc.Natl.Acad.Sci.USA,
120, e2304378120. doi: 10.1073/pnas.2304378120.
- Abstract
- ToxR, a <i>Vibrio cholerae</i>
transmembrane one-component signal transduction factor,
lies within a regulatory cascade that results in the
expression of ToxT, toxin coregulated pilus, and cholera
toxin. While ToxR has been extensively studied for its
ability to activate or repress various genes in <i>V.
cholerae</i>, here we present the crystal structures
of the ToxR cytoplasmic domain bound to DNA at the
<i>toxT</i> and <i>ompU</i>
promoters. The structures confirm some predicted
interactions, yet reveal other unexpected promoter
interactions with implications for other potential
regulatory roles for ToxR. We show that ToxR is a versatile
virulence regulator that recognizes diverse and extensive,
eukaryotic-like regulatory DNA sequences, that relies more
on DNA structural elements than specific sequences for
binding. Using this topological DNA recognition mechanism,
ToxR can bind both in tandem and in a twofold
inverted-repeat-driven manner. Its regulatory action is
based on coordinated multiple binding to promoter regions
near the transcription start site, which can remove the
repressing H-NS proteins and prepares the DNA for optimal
interaction with the RNA polymerase.