Summary information and primary citation
- PDB-id
-
7zah;
SNAP-derived features in text and
JSON formats
- Class
- translation
- Method
- cryo-EM (2.7 Å)
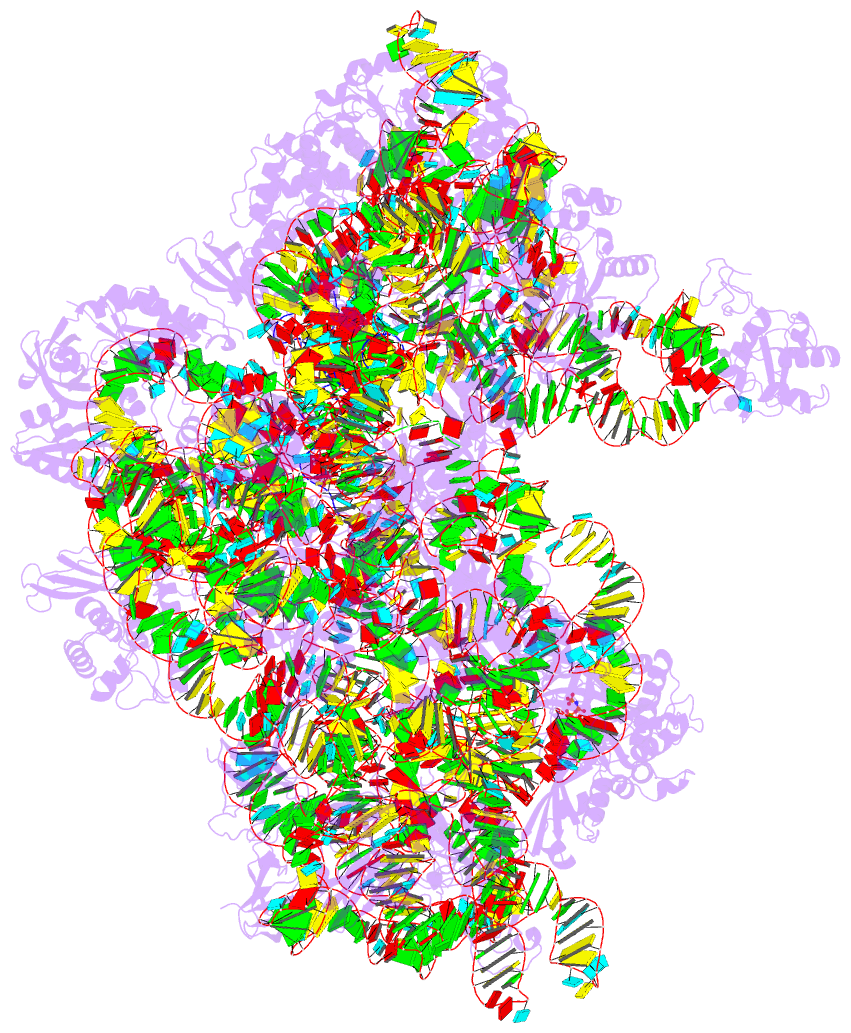
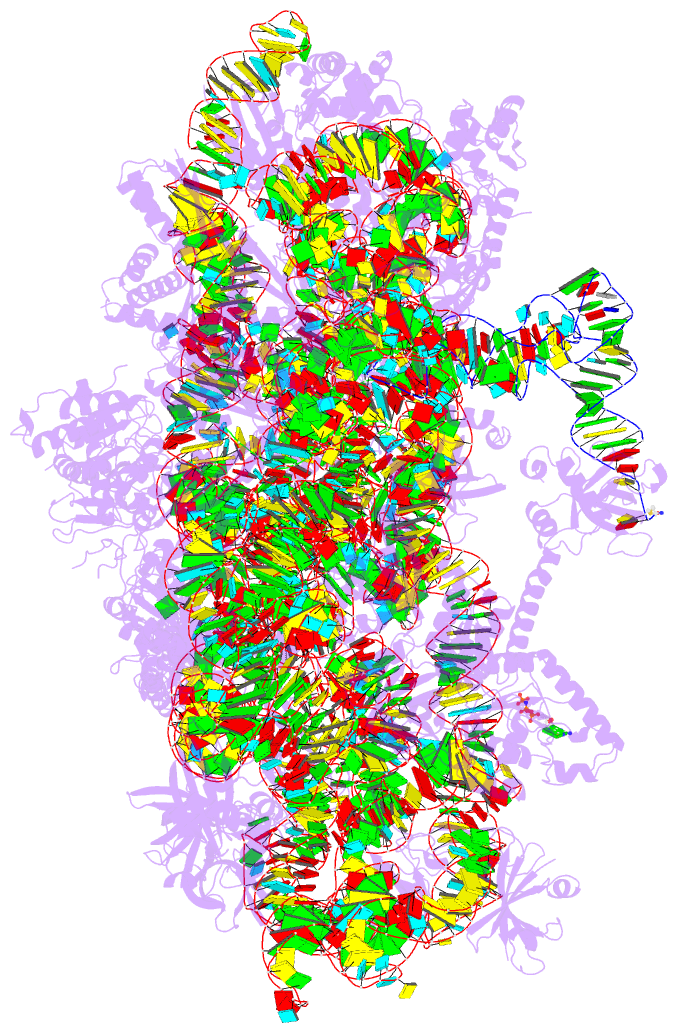
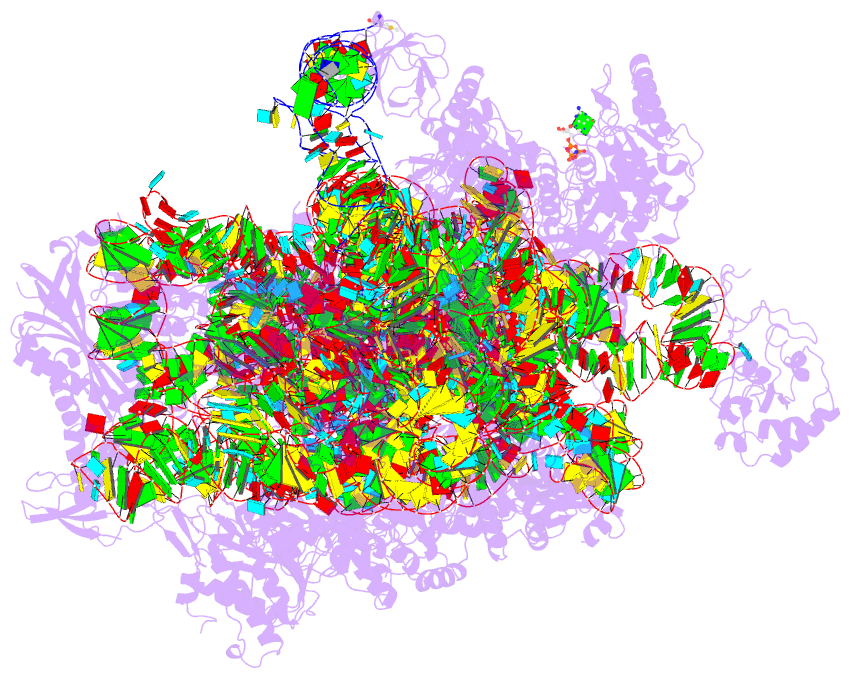
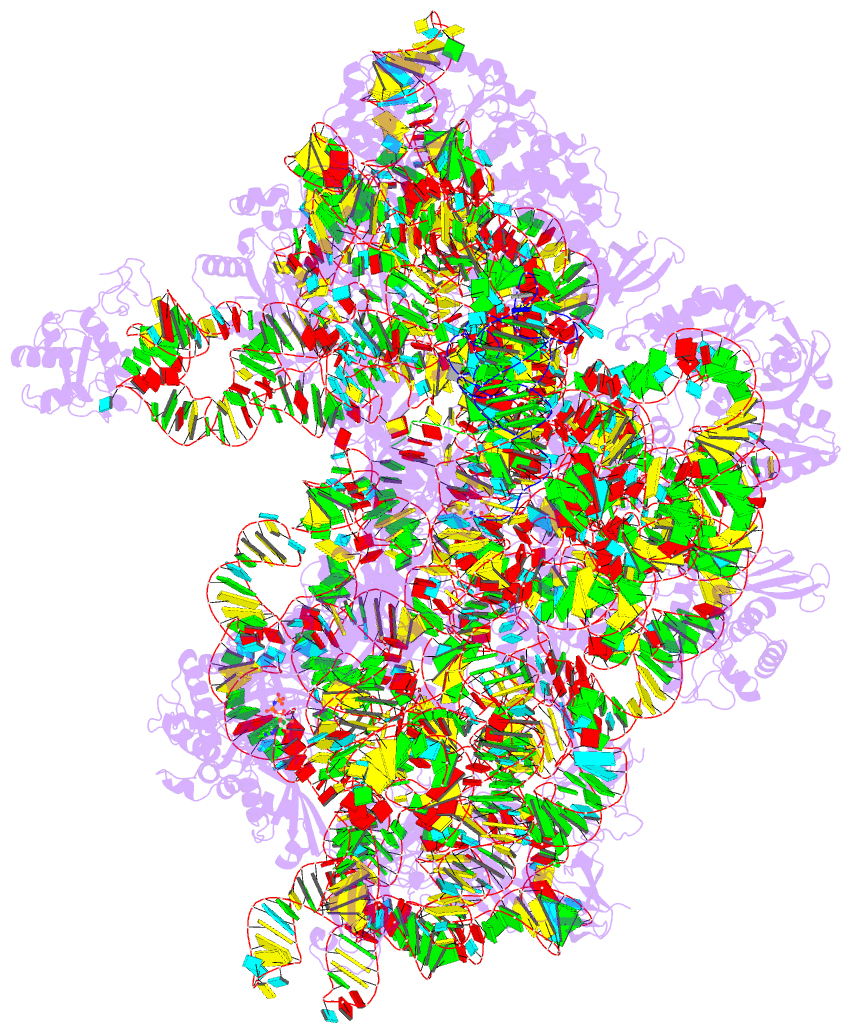
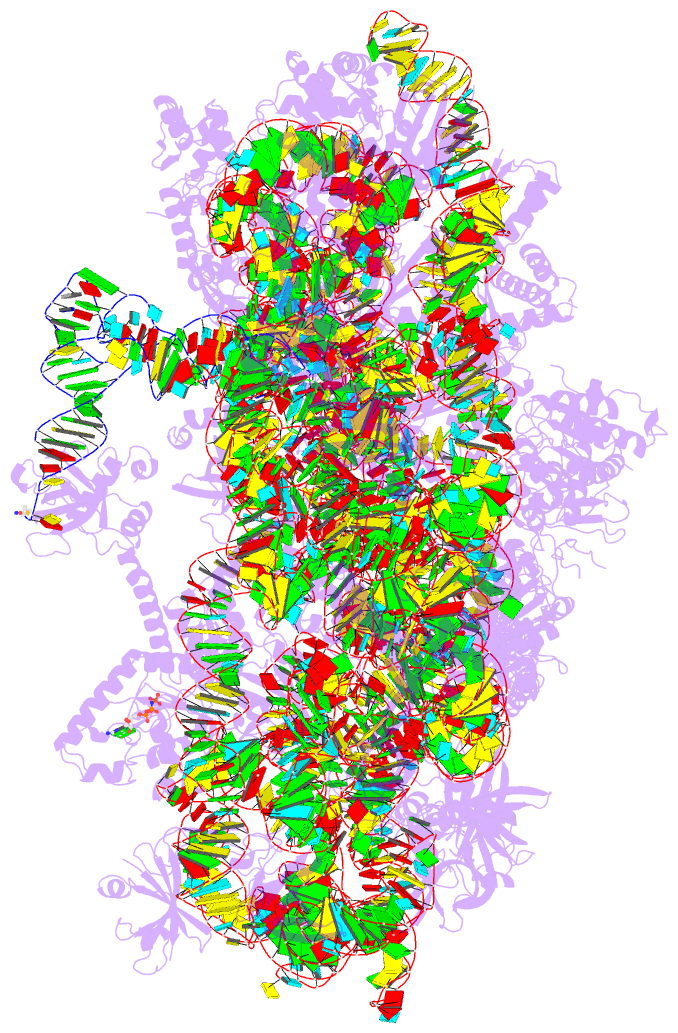
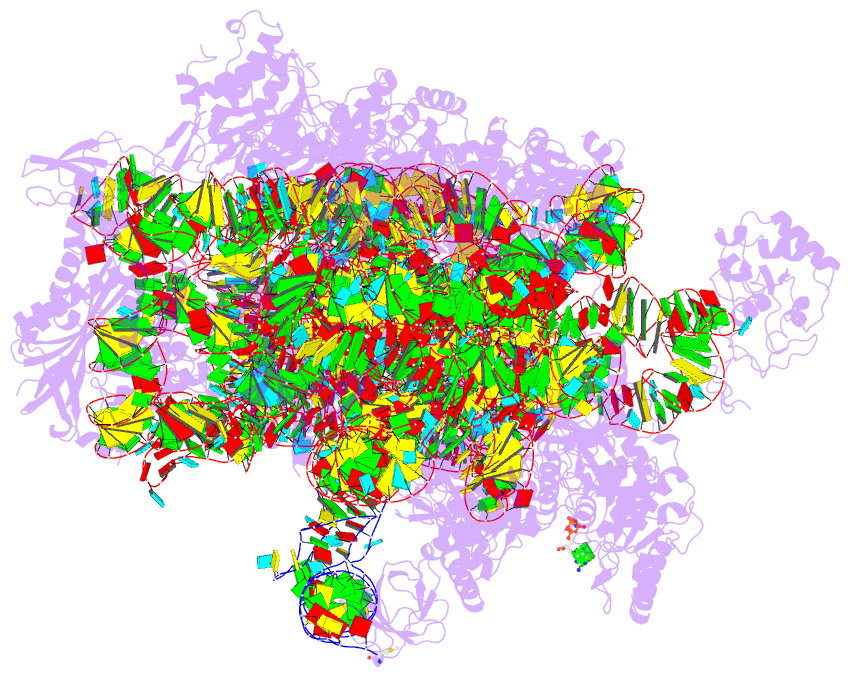
- Summary
- cryo-EM structure of a pyrococcus abyssi 30s bound to
met-initiator trna, mrna, aif1a and aif5b
- Reference
-
Kazan R, Bourgeois G, Lazennec-Schurdevin C, Larquet E,
Mechulam Y, Coureux PD, Schmitt E (2022): "Role of
aIF5B in archaeal translation initiation."
Nucleic Acids Res., 50,
6532-6548. doi: 10.1093/nar/gkac490.
- Abstract
- In eukaryotes and in archaea late steps of translation
initiation involve the two initiation factors e/aIF5B and
e/aIF1A. In eukaryotes, the role of eIF5B in ribosomal
subunit joining is established and structural data showing
eIF5B bound to the full ribosome were obtained. To achieve
its function, eIF5B collaborates with eIF1A. However,
structural data illustrating how these two factors interact
on the small ribosomal subunit have long been awaited. The
role of the archaeal counterparts, aIF5B and aIF1A, remains
to be extensively addressed. Here, we study the late steps
of Pyrococcus abyssi translation initiation. Using in vitro
reconstituted initiation complexes and light scattering, we
show that aIF5B bound to GTP accelerates subunit joining
without the need for GTP hydrolysis. We report the
crystallographic structures of aIF5B bound to GDP and GTP
and analyze domain movements associated to these two
nucleotide states. Finally, we present the cryo-EM
structure of an initiation complex containing 30S bound to
mRNA, Met-tRNAiMet, aIF5B and aIF1A at 2.7 Å resolution.
Structural data shows how archaeal 5B and 1A factors
cooperate to induce a conformation of the initiator tRNA
favorable to subunit joining. Archaeal and eukaryotic
features of late steps of translation initiation are
discussed.