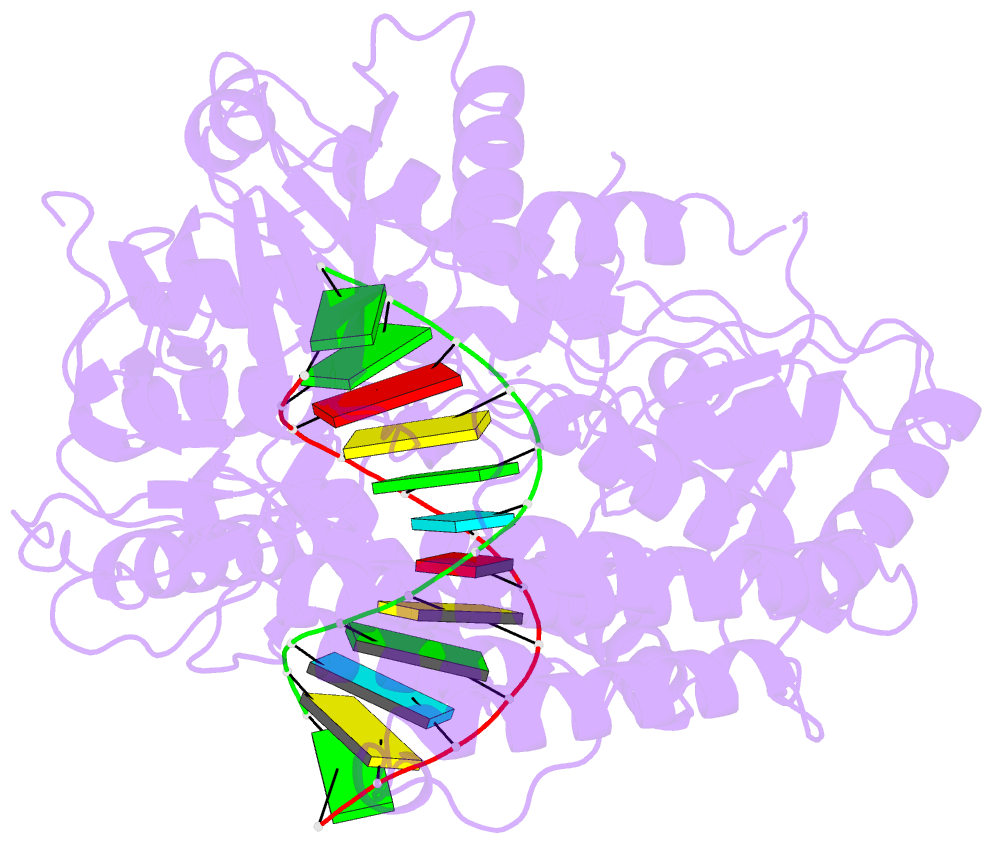
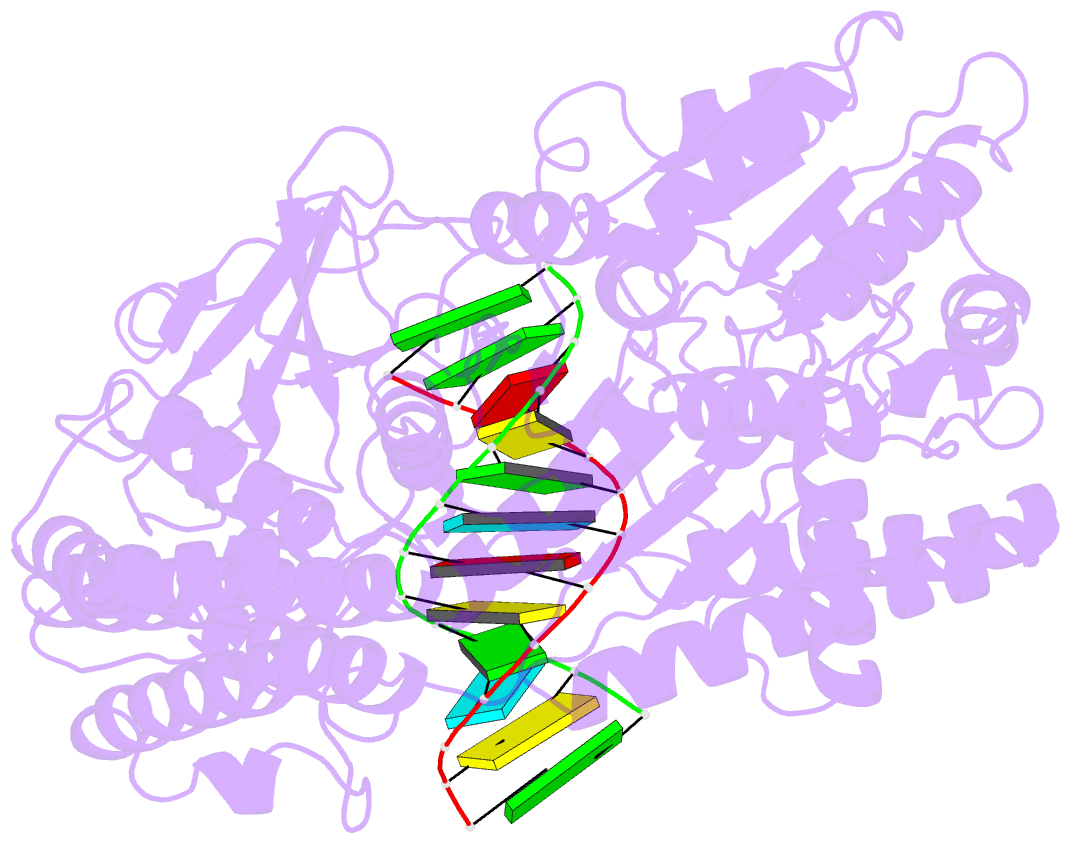
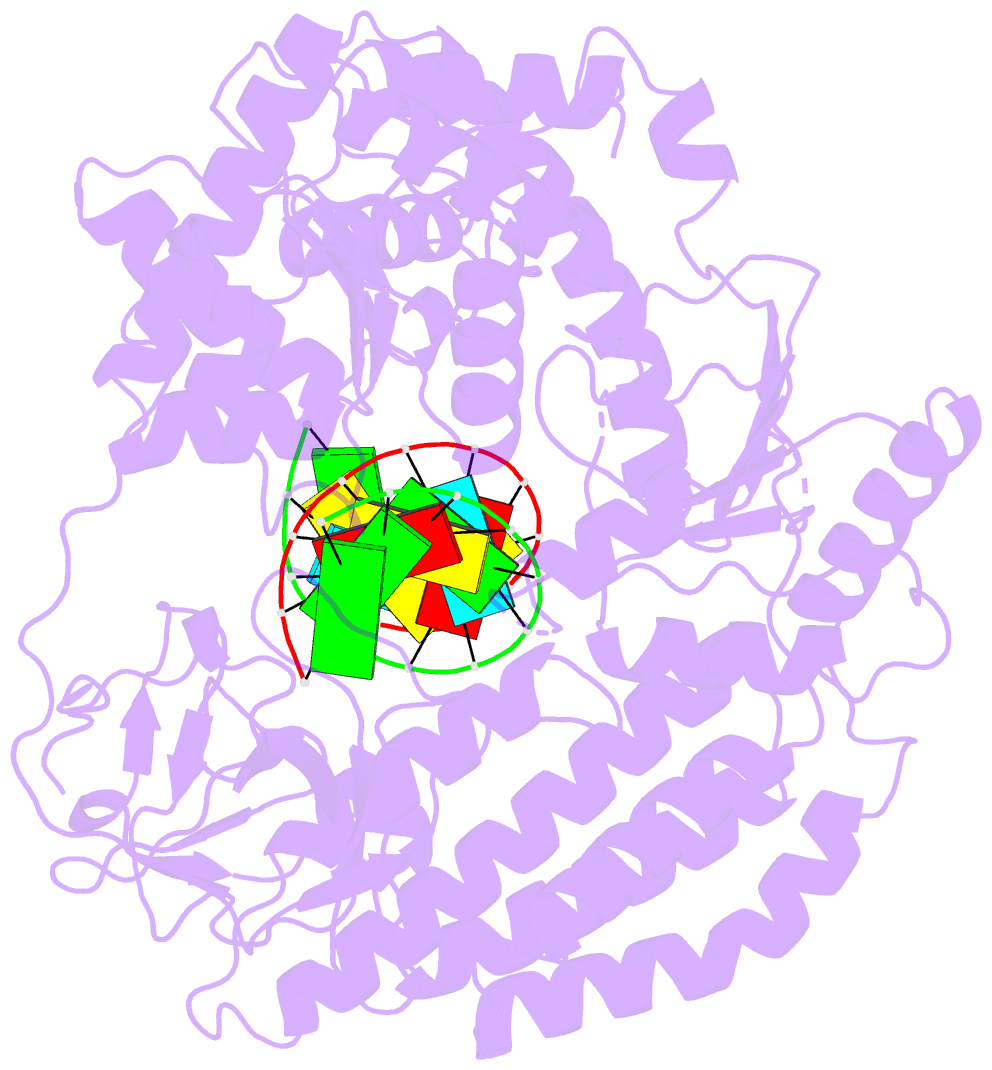
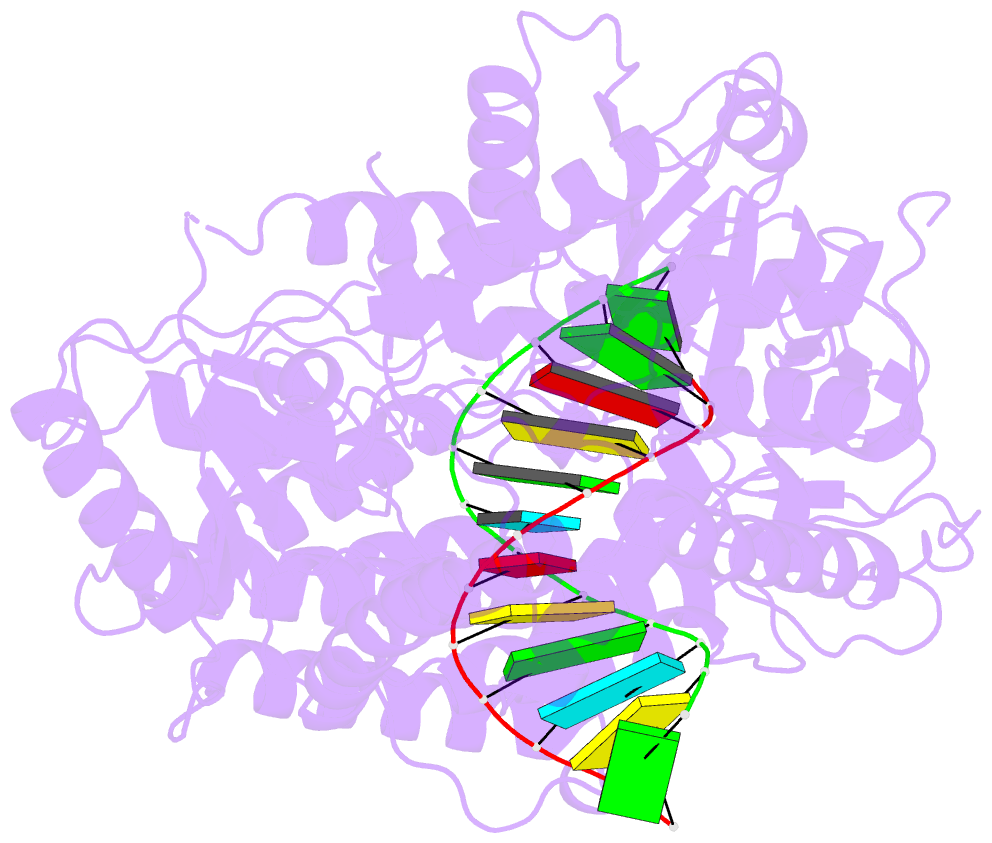
Summary information and primary citation
- PDB-id
-
7tny;
SNAP-derived features in text and
JSON formats
- Class
- immune system-RNA
- Method
- cryo-EM (3.2 Å)
- Summary
- cryo-EM structure of rig-i in complex with p2dsrna
- Reference
-
Wang W, Pyle AM (2022): "The RIG-I
receptor adopts two different conformations for
distinguishing host from viral RNA ligands."
Mol.Cell, 82, 4131. doi:
10.1016/j.molcel.2022.09.029.
- Abstract
- RIG-I is an essential innate immune receptor for
detecting and responding to infection by RNA viruses. RIG-I
specifically recognizes the unique molecular features of
viral RNA molecules and selectively distinguishes them from
closely related RNAs abundant in host cells. The physical
basis for this exquisite selectivity is revealed through a
series of high-resolution cryo-EM structures of RIG-I in
complex with host and viral RNA ligands. These studies
demonstrate that RIG-I actively samples double-stranded
RNAs in the cytoplasm and distinguishes them by adopting
two different types of protein folds. Upon binding viral
RNA, RIG-I adopts a high-affinity conformation that is
conducive to signaling, while host RNA induces an
autoinhibited conformation that stimulates RNA release. By
coupling protein folding with RNA binding selectivity,
RIG-I distinguishes RNA molecules that differ by as little
as one phosphate group, thereby explaining the molecular
basis for selective antiviral sensing and the induction of
autoimmunity upon RIG-I dysregulation.