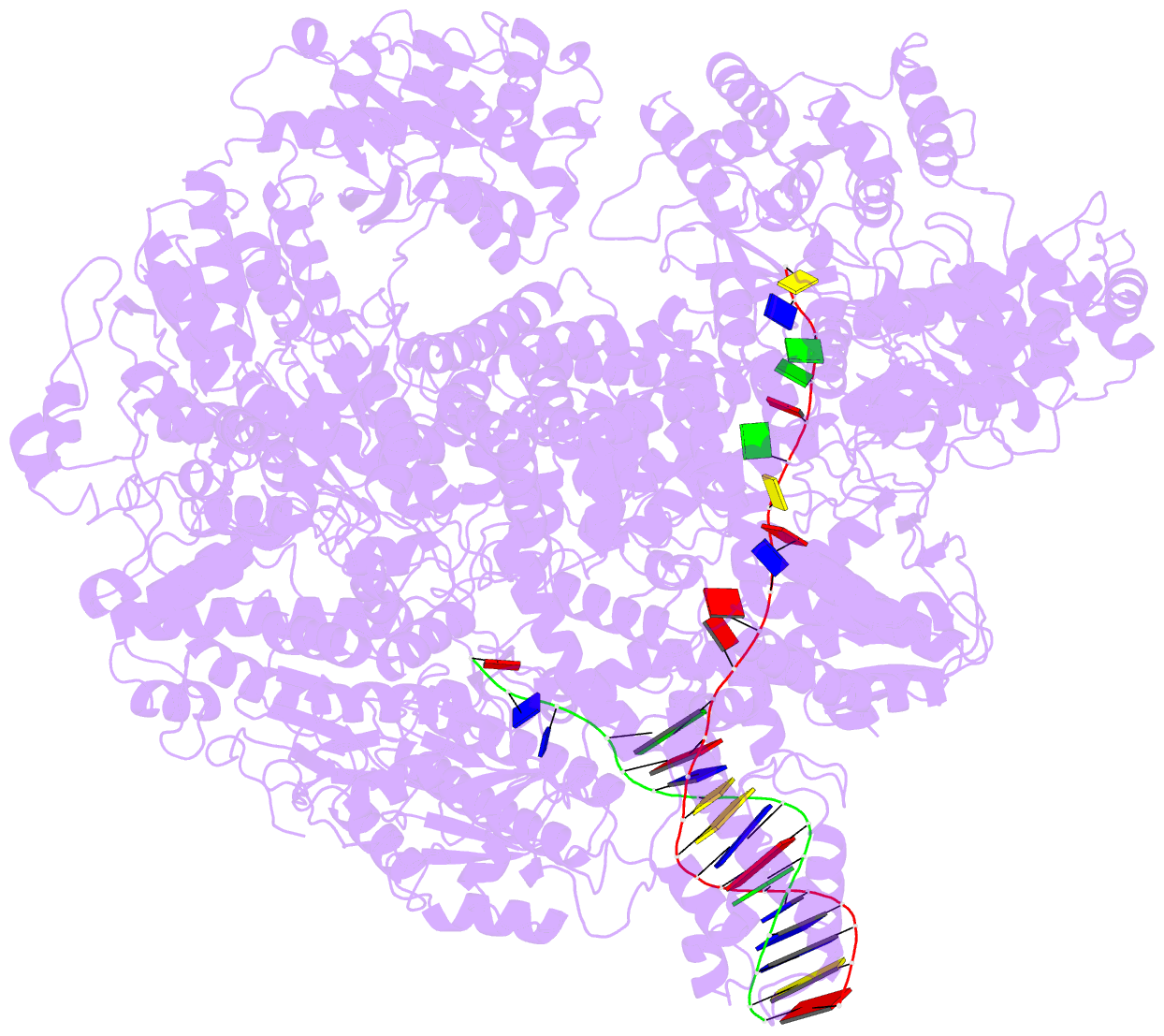
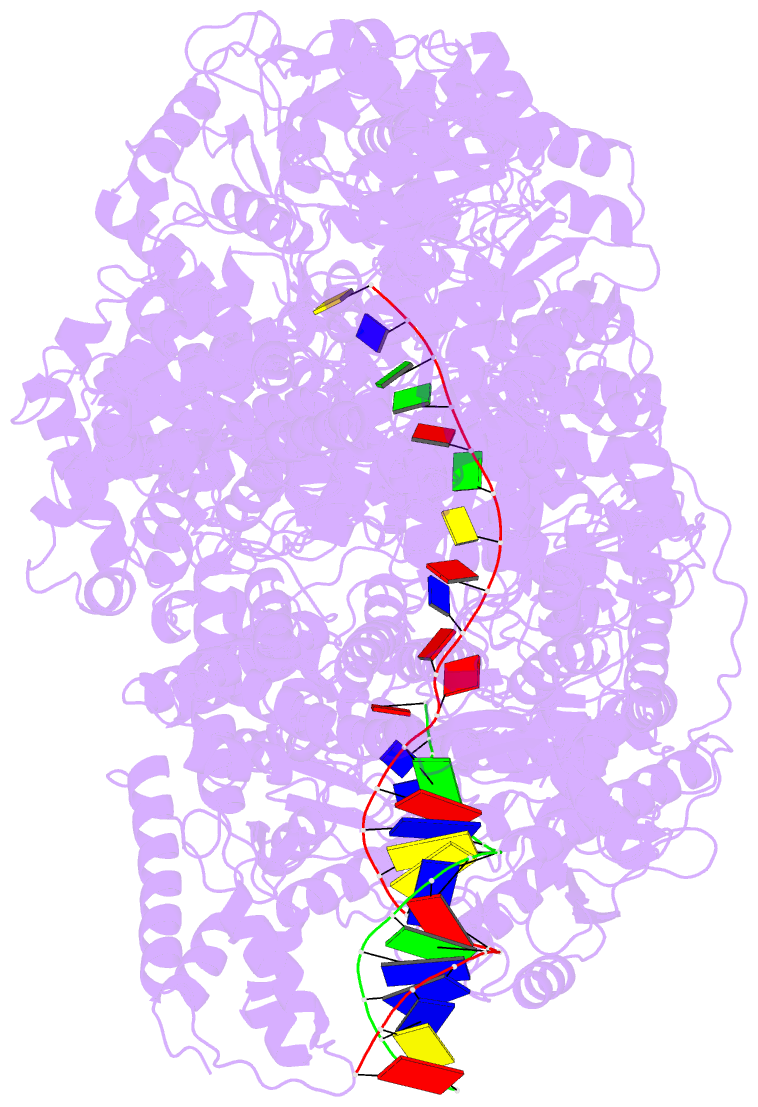
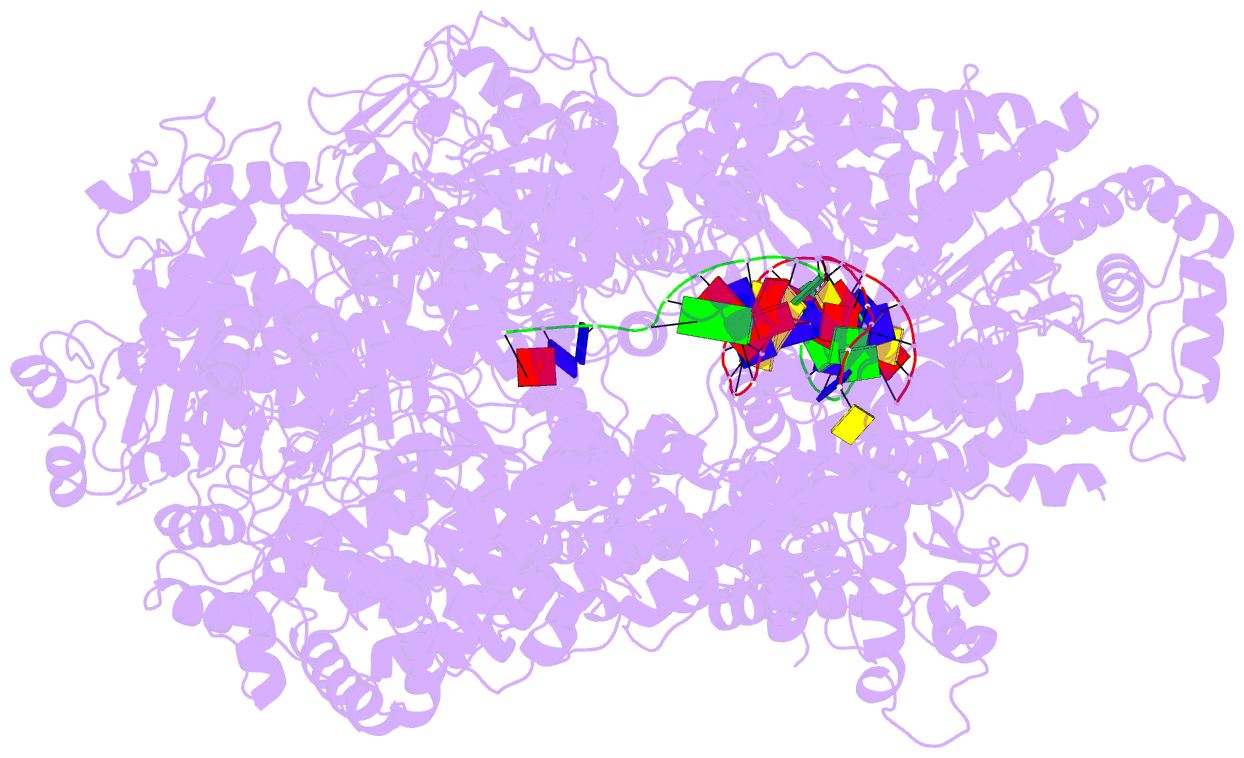
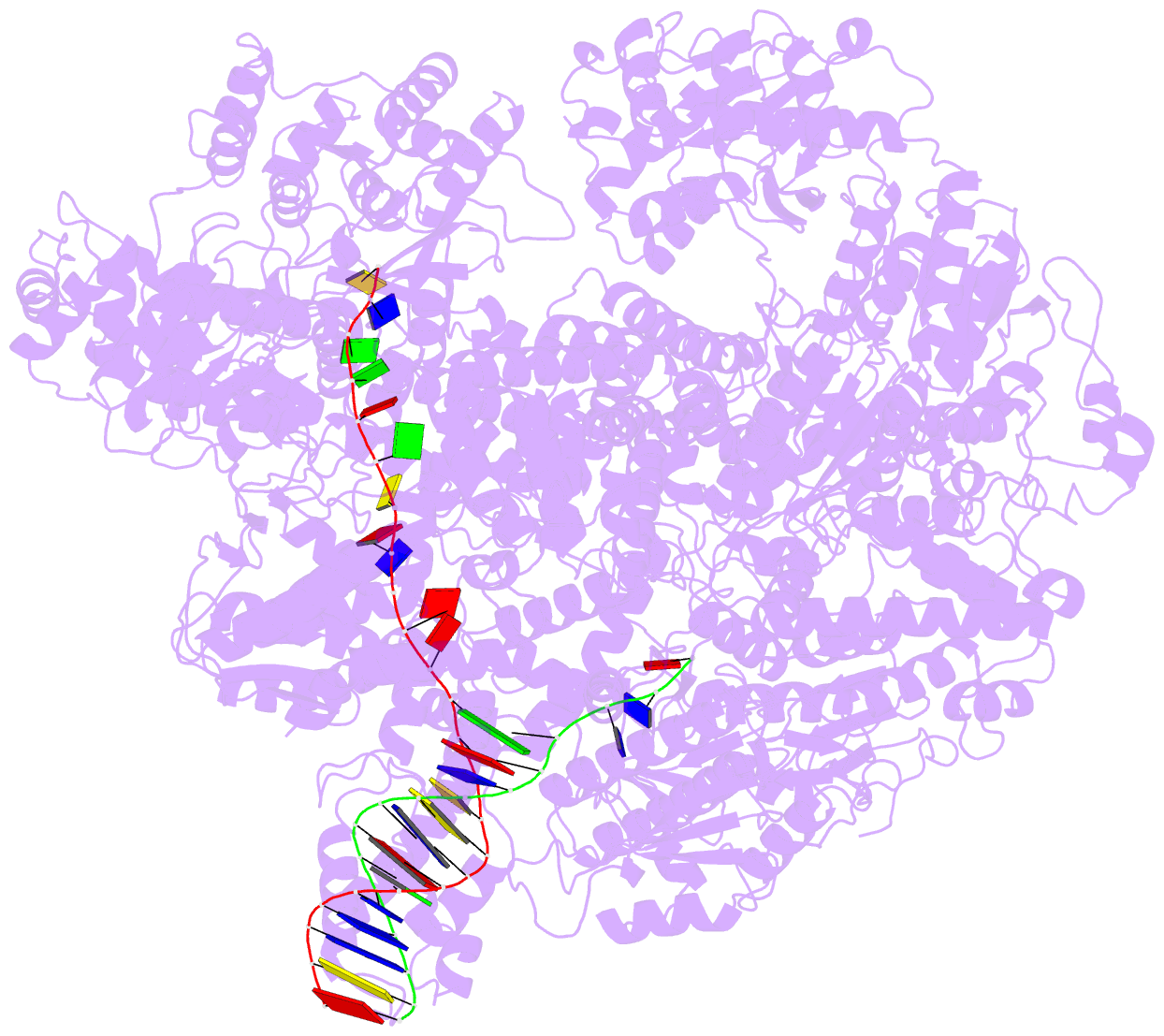
Summary information and primary citation
- PDB-id
- 7mr3; DSSR-derived features in text and JSON formats
- Class
- hydrolase-DNA
- Method
- cryo-EM (3.6 Å)
- Summary
- cryo-EM structure of recbcd-DNA complex with docked recbnuc and stabilized recd
- Reference
- Hao L, Zhang R, Lohman TM (2021): "Heterogeneity in E. coli RecBCD Helicase-DNA binding and base pair melting." J.Mol.Biol., 167147. doi: 10.1016/j.jmb.2021.167147.
- Abstract
- E. coli RecBCD, a helicase/nuclease involved in double stranded (ds) DNA break repair, binds to a dsDNA end and melts out several DNA base pairs (bp) using only its binding free energy. We examined RecBCD-DNA initiation complexes using thermodynamic and structural approaches. Measurements of enthalpy changes for RecBCD binding to DNA ends possessing pre-melted ssDNA tails of increasing length suggest that RecBCD interacts with ssDNA as long as 17-18 nucleotides and can melt at least 10-11 bp upon binding a blunt DNA end. Cryo-EM structures of RecBCD alone and in complex with a blunt-ended dsDNA show significant conformational heterogeneities associated with the RecB nuclease domain (RecBNuc) and the RecD subunit. In the absence of DNA, 56% of RecBCD molecules show no density for the RecB nuclease domain, RecBNuc, and all RecBCD molecules show only partial density for RecD. DNA binding reduces these conformational heterogeneities, with 63% of the molecules showing density for both RecD and RecBNuc. This suggests that the RecBNuc domain is dynamic and influenced by DNA binding. The major RecBCD-DNA structural class in which RecBNuc is docked onto RecC shows melting of at least 11 bp from a blunt DNA end, much larger than previously observed. A second structural class in which RecBNuc is not docked shows only four bp melted suggesting that RecBCD complexes transition between states with different extents of DNA melting and that the extent of melting regulates initiation of helicase activity.