Summary information and primary citation
- PDB-id
- 6xla; DSSR-derived features in text and JSON formats
- Class
- transcription
- Method
- cryo-EM (3.1 Å)
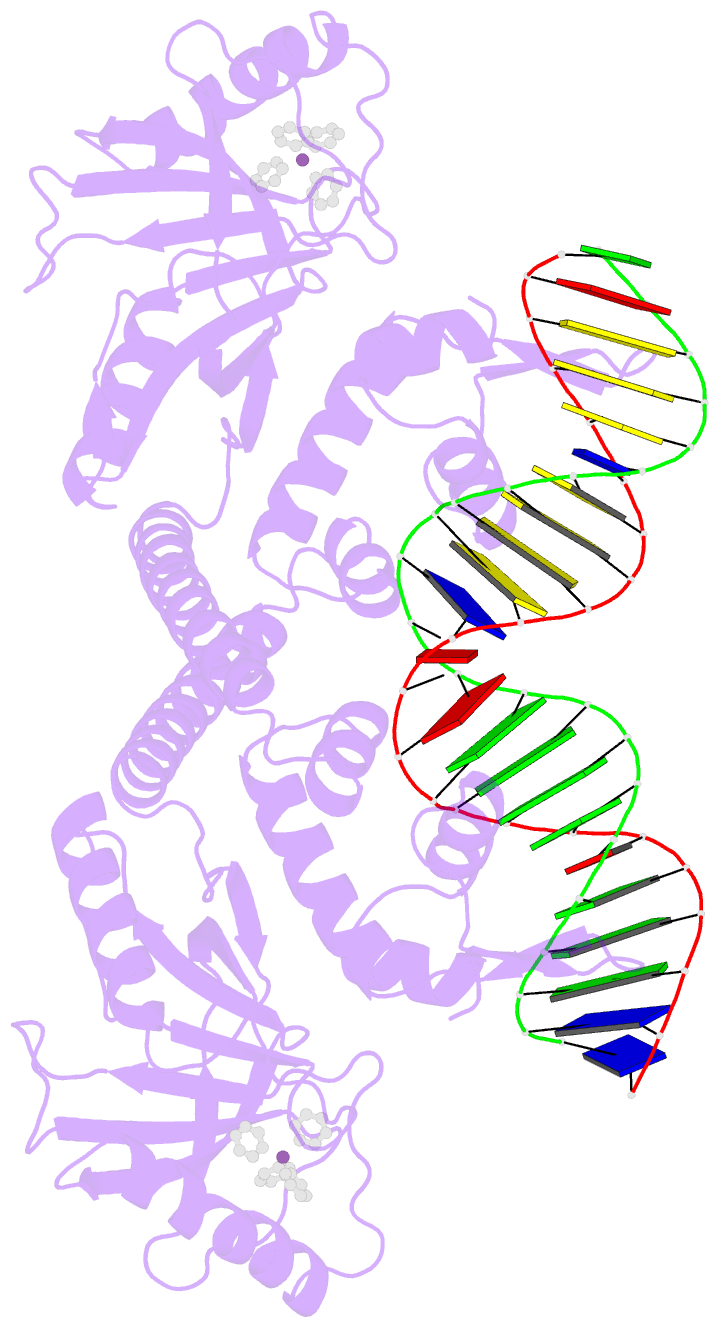
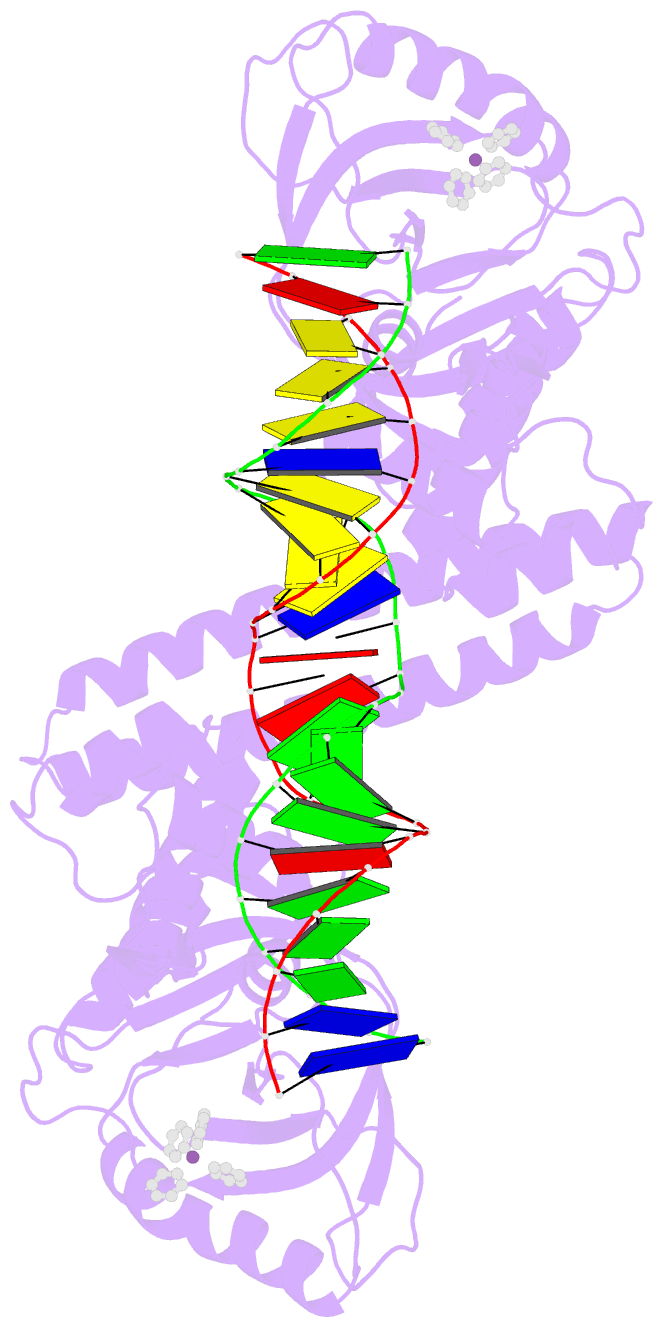
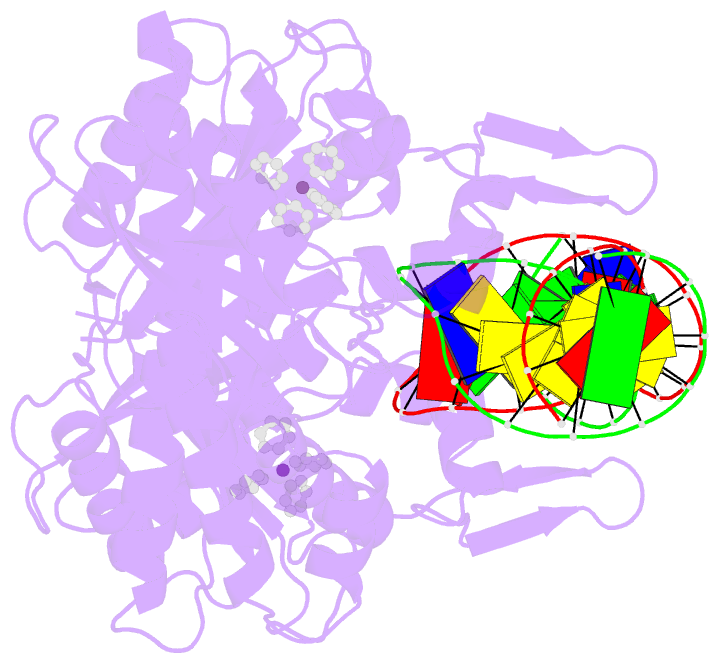
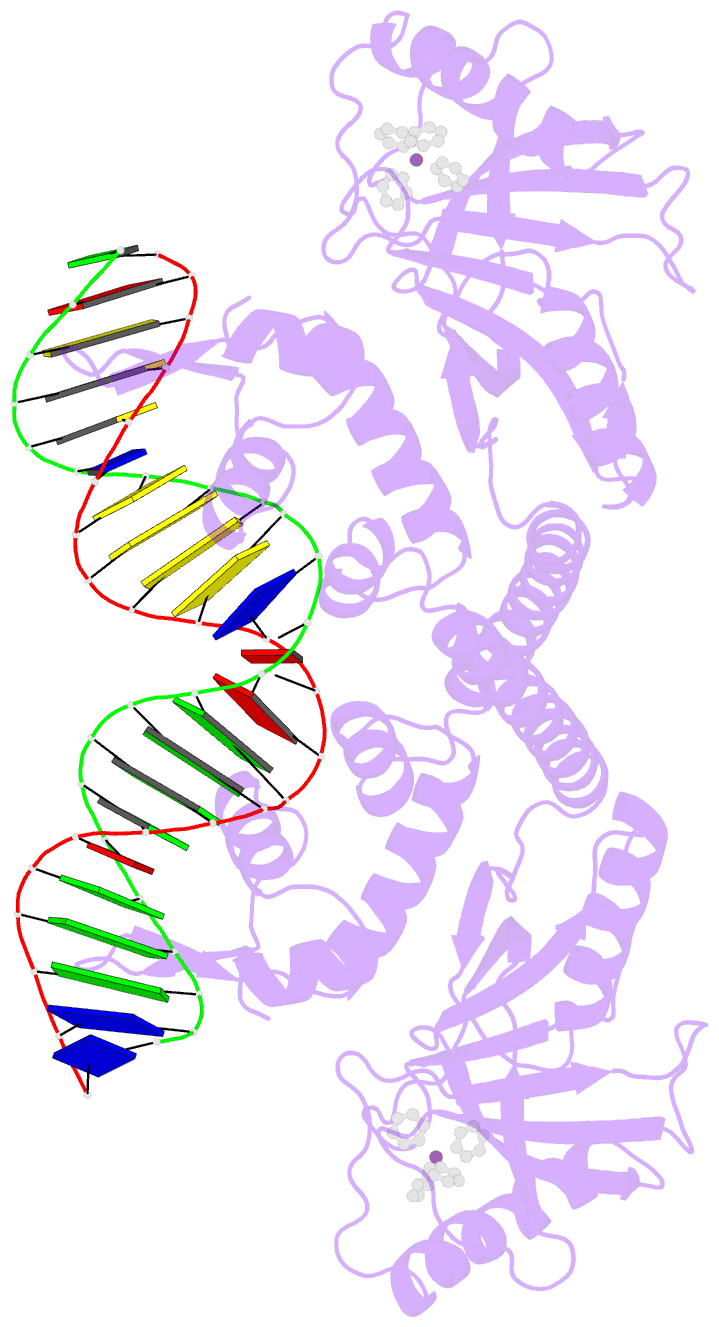
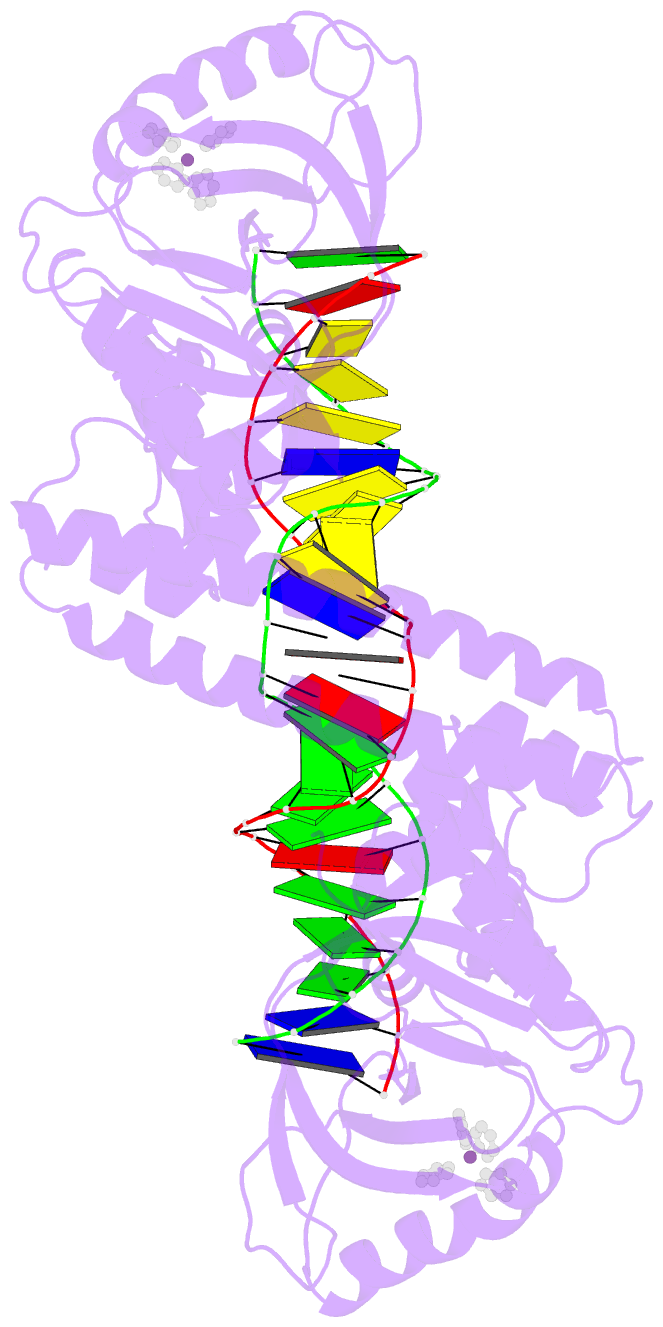
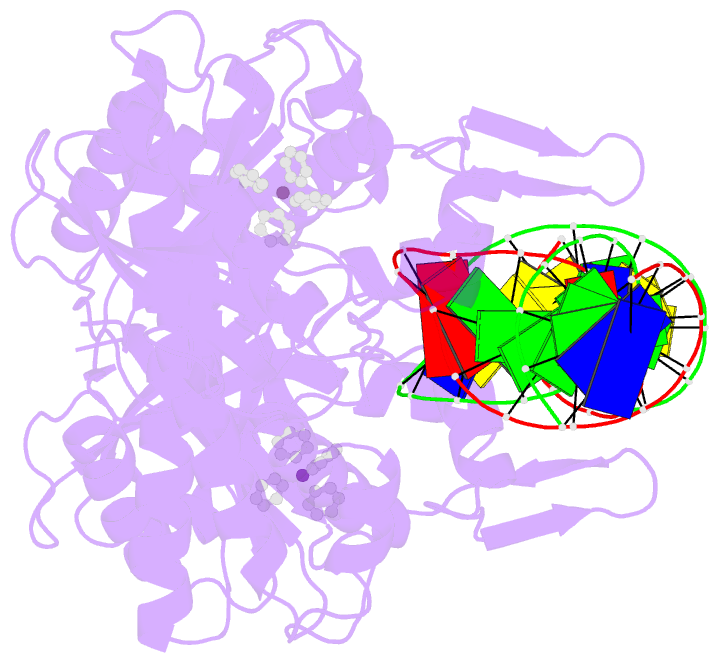
- Summary
- cryo-EM structure of ecmrr-DNA complex in ecmrr-rpitc-3nt
- Reference
- Yang Y, Liu C, Zhou W, Shi W, Chen M, Zhang B, Schatz DG, Hu Y, Liu B (2021): "Structural visualization of transcription activated by a multidrug-sensing MerR family regulator." Nat Commun, 12, 2702. doi: 10.1038/s41467-021-22990-8.
- Abstract
- Bacterial RNA polymerase (RNAP) holoenzyme initiates transcription by recognizing the conserved -35 and -10 promoter elements that are optimally separated by a 17-bp spacer. The MerR family of transcriptional regulators activate suboptimal 19-20 bp spacer promoters in response to myriad cellular signals, ranging from heavy metals to drug-like compounds. The regulation of transcription by MerR family regulators is not fully understood. Here we report one crystal structure of a multidrug-sensing MerR family regulator EcmrR and nine cryo-electron microscopy structures that capture the EcmrR-dependent transcription process from promoter opening to initial transcription to RNA elongation. These structures reveal that EcmrR is a dual ligand-binding factor that reshapes the suboptimal 19-bp spacer DNA to enable optimal promoter recognition, sustains promoter remodeling to stabilize initial transcribing complexes, and finally dissociates from the promoter to reverse DNA remodeling and facilitate the transition to elongation. Our findings yield a comprehensive model for transcription regulation by MerR family factors and provide insights into the transition from transcription initiation to elongation.