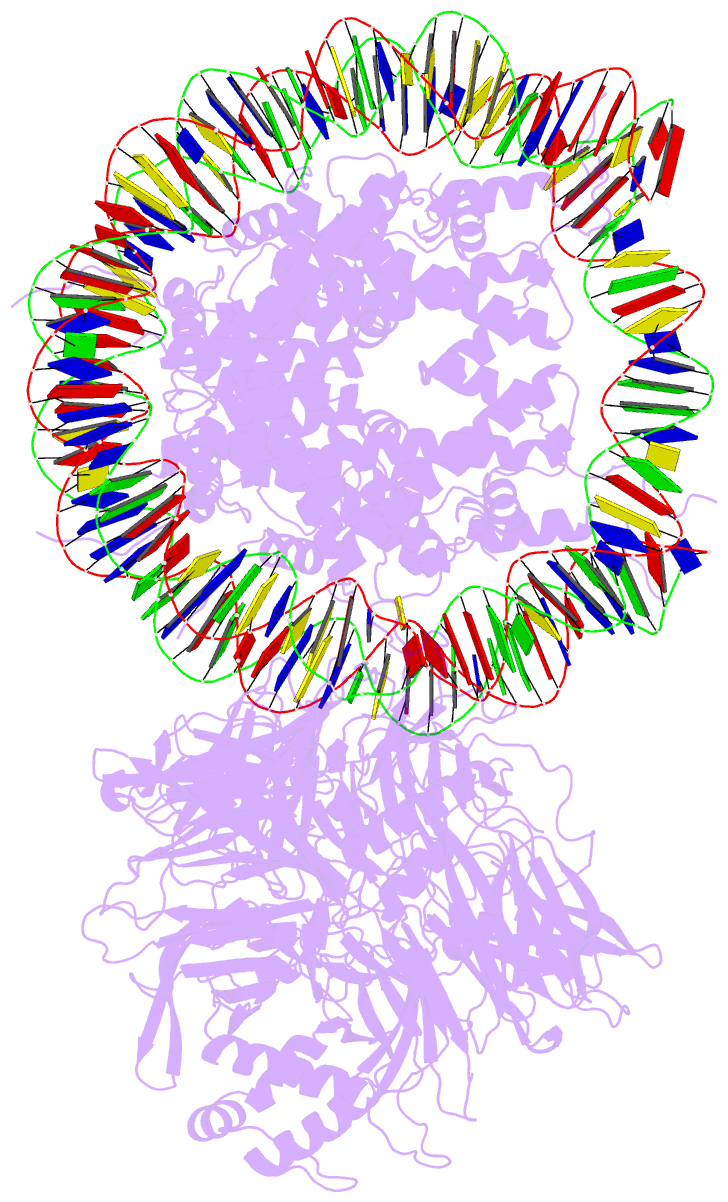
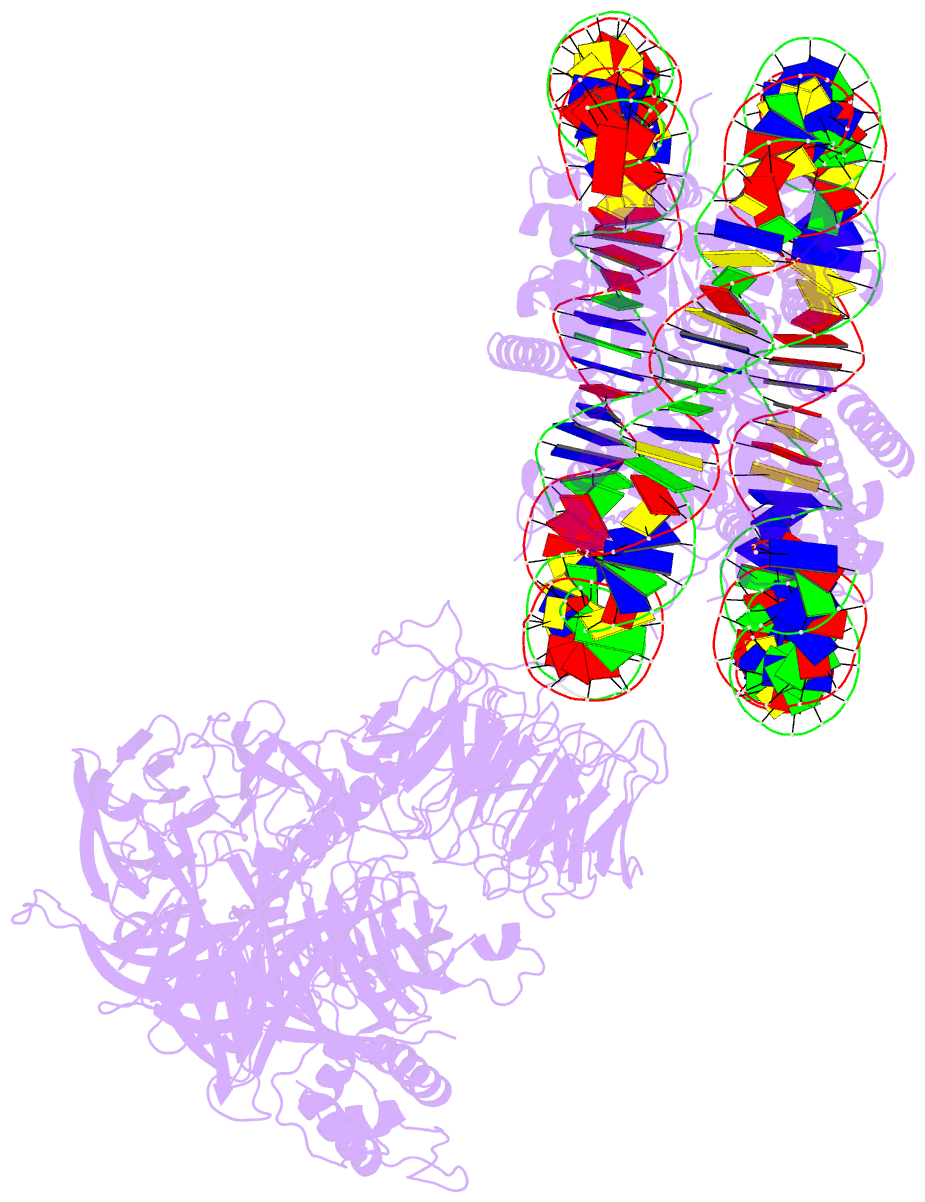
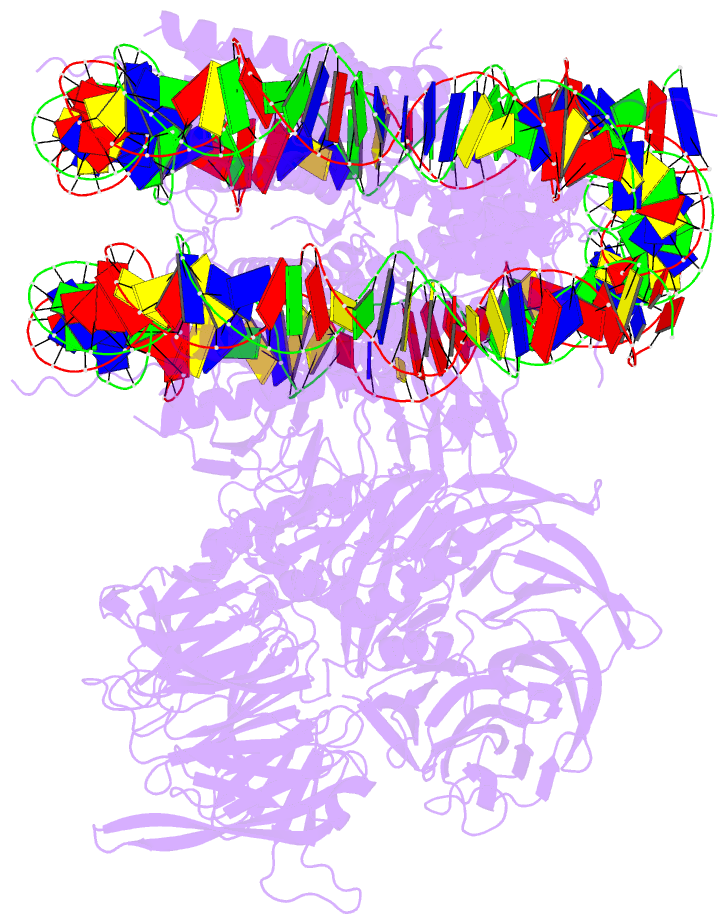
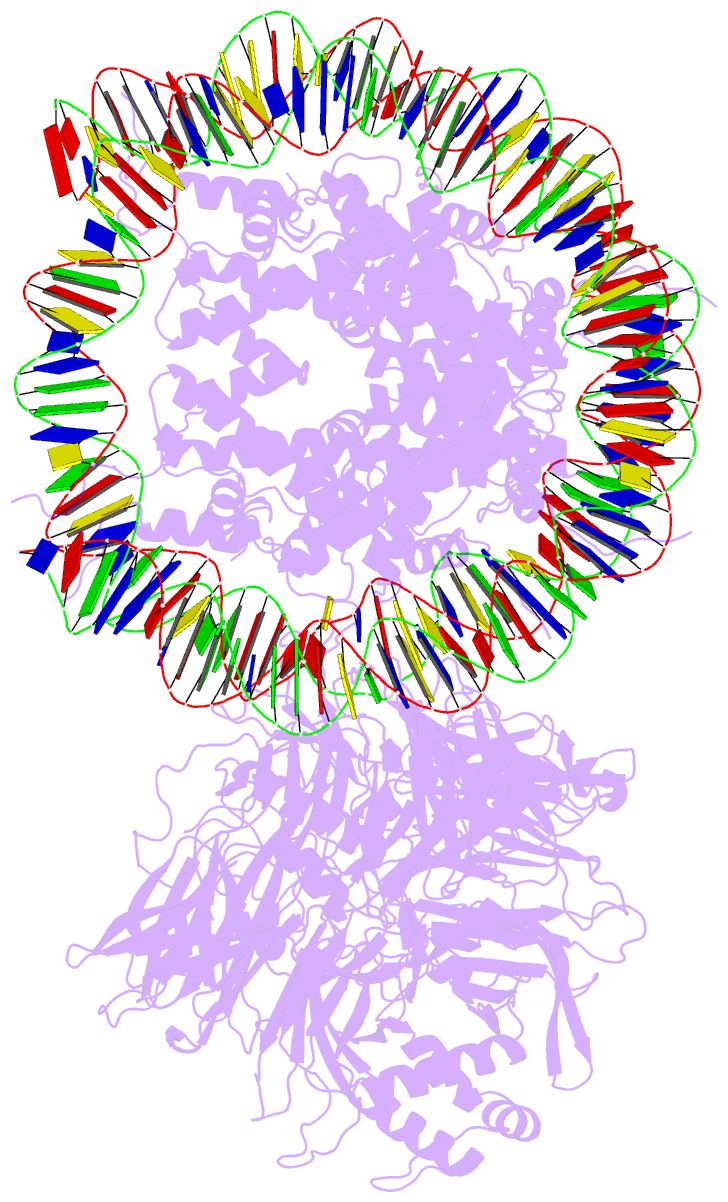
Summary information and primary citation
- PDB-id
- 6r92; DSSR-derived features in text and JSON formats
- Class
- DNA binding protein
- Method
- cryo-EM (4.8 Å)
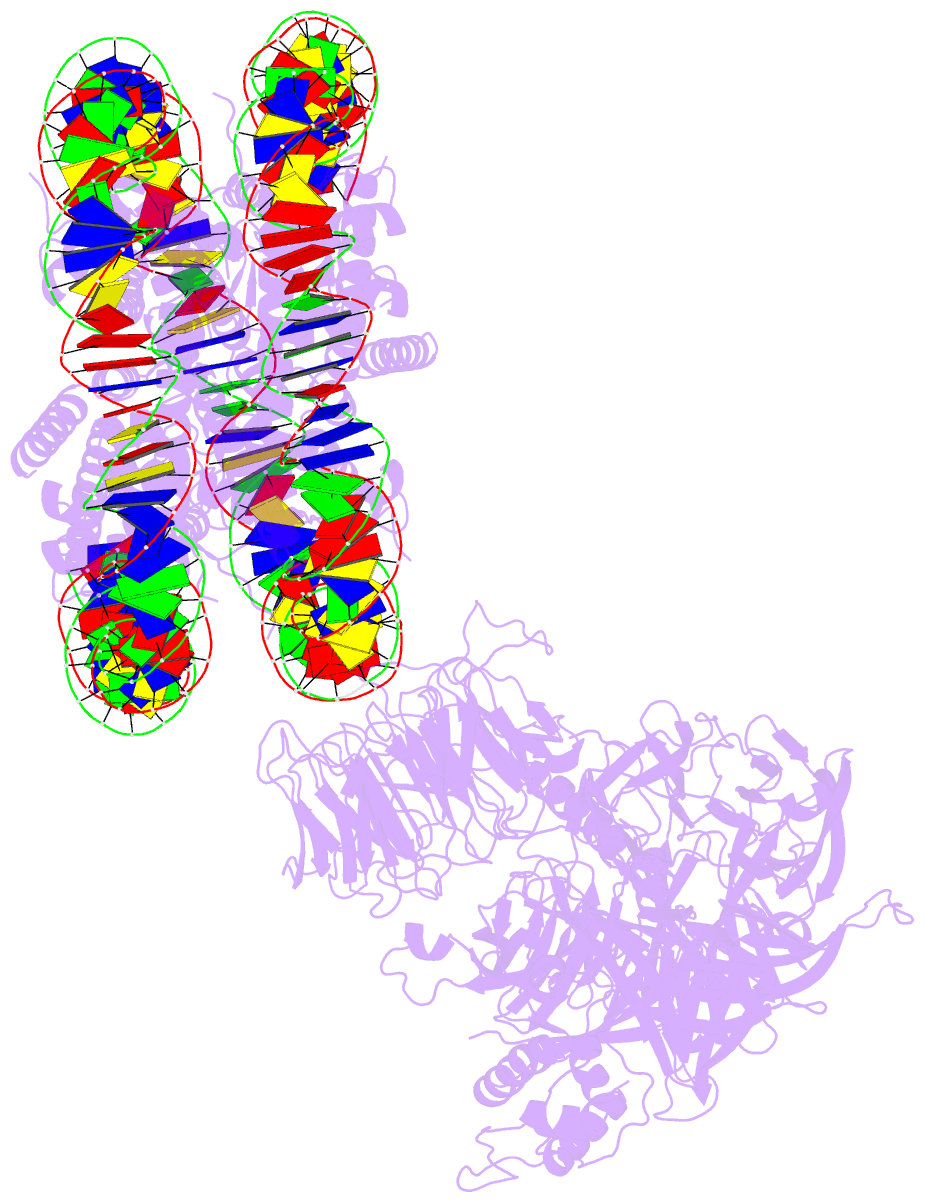
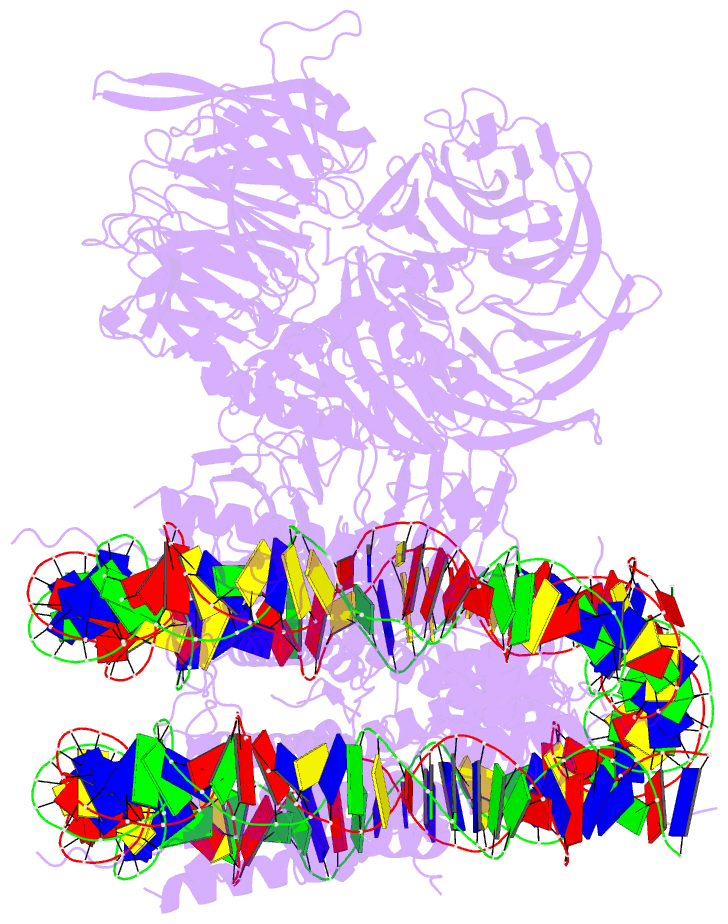
- Summary
- cryo-EM structure of ncp-thf2(+1)-uv-ddb class b
- Reference
- Matsumoto S, Cavadini S, Bunker RD, Grand RS, Potenza A, Rabl J, Yamamoto J, Schenk AD, Schubeler D, Iwai S, Sugasawa K, Kurumizaka H, Thoma NH (2019): "DNA damage detection in nucleosomes involves DNA register shifting." Nature, 571, 79-84. doi: 10.1038/s41586-019-1259-3.
- Abstract
- Access to DNA packaged in nucleosomes is critical for gene regulation, DNA replication and DNA repair. In humans, the UV-damaged DNA-binding protein (UV-DDB) complex detects UV-light-induced pyrimidine dimers throughout the genome; however, it remains unknown how these lesions are recognized in chromatin, in which nucleosomes restrict access to DNA. Here we report cryo-electron microscopy structures of UV-DDB bound to nucleosomes bearing a 6-4 pyrimidine-pyrimidone dimer or a DNA-damage mimic in various positions. We find that UV-DDB binds UV-damaged nucleosomes at lesions located in the solvent-facing minor groove without affecting the overall nucleosome architecture. In the case of buried lesions that face the histone core, UV-DDB changes the predominant translational register of the nucleosome and selectively binds the lesion in an accessible, exposed position. Our findings explain how UV-DDB detects occluded lesions in strongly positioned nucleosomes, and identify slide-assisted site exposure as a mechanism by which high-affinity DNA-binding proteins can access otherwise occluded sites in nucleosomal DNA.