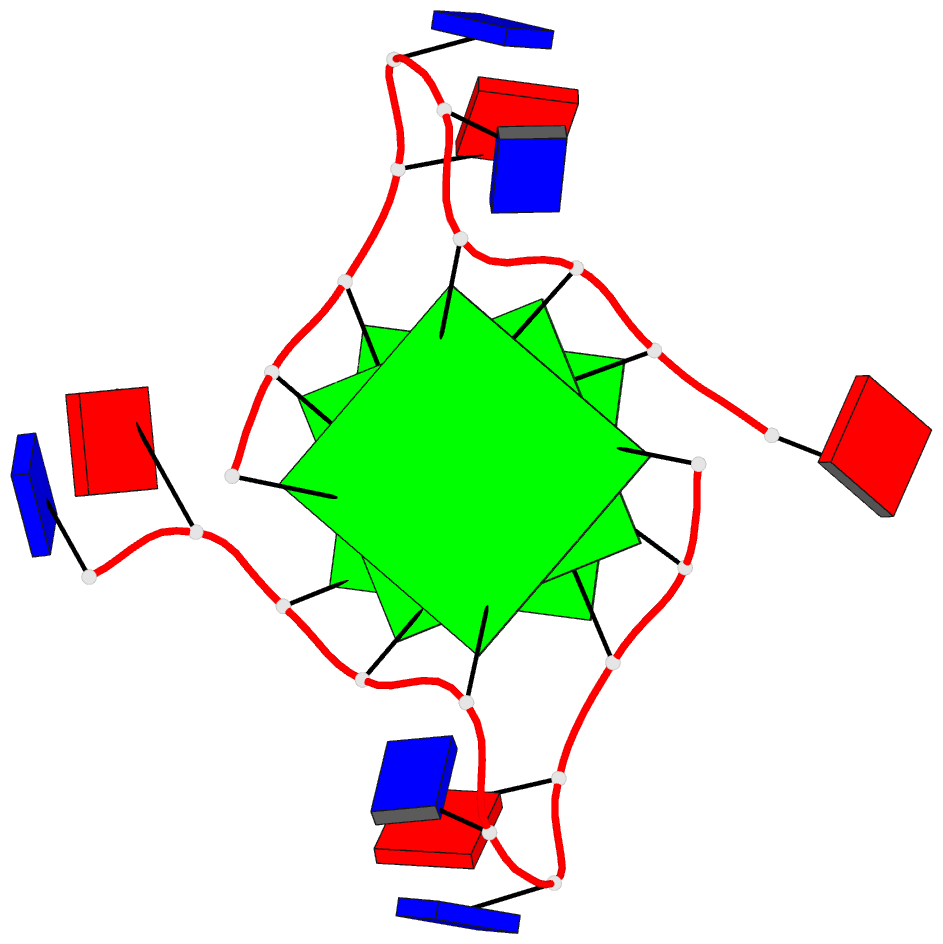
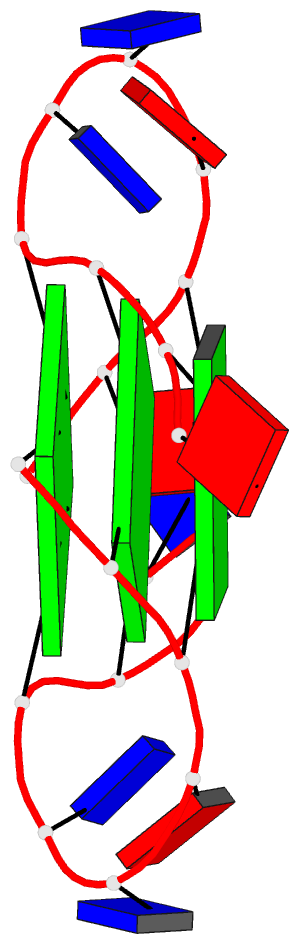
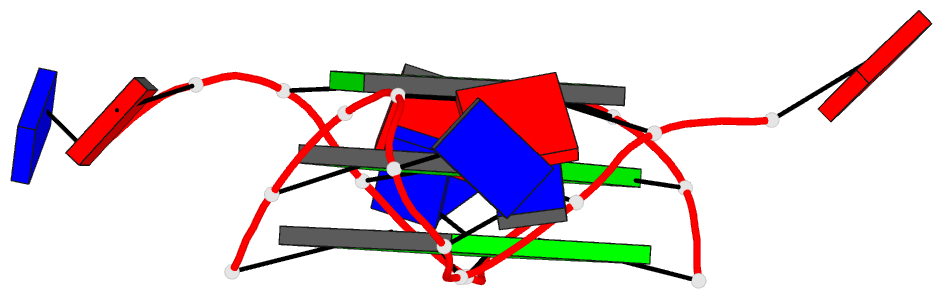
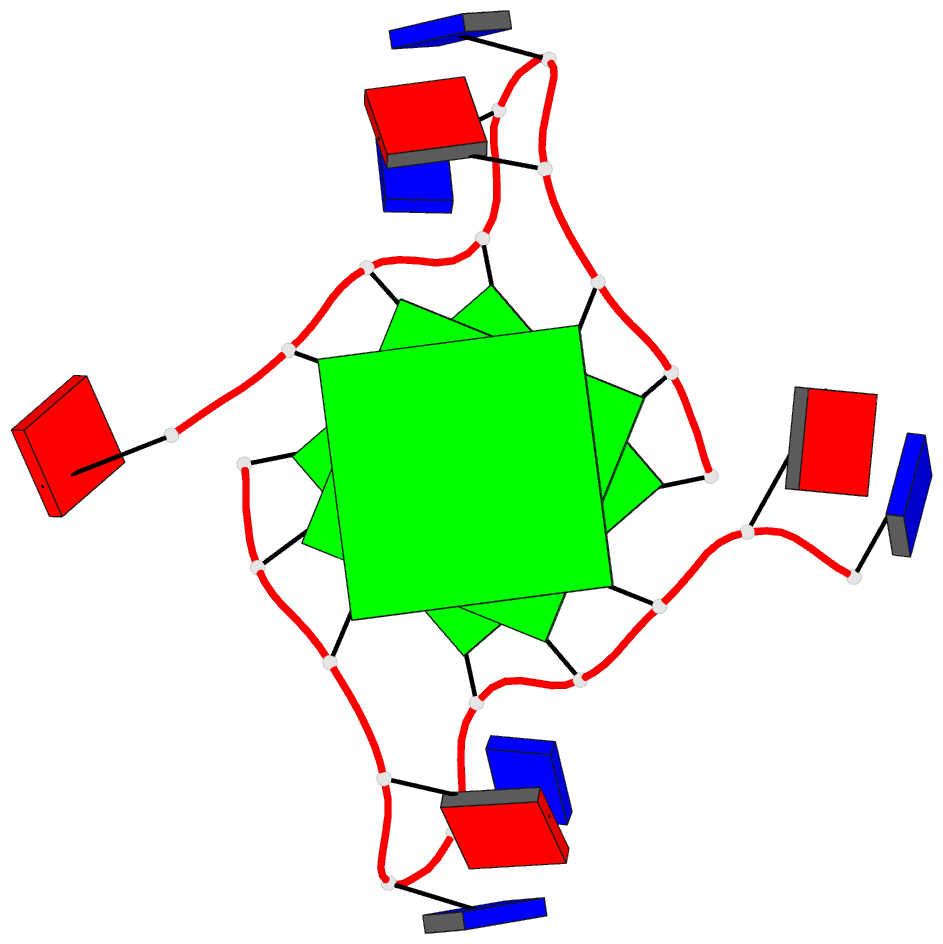
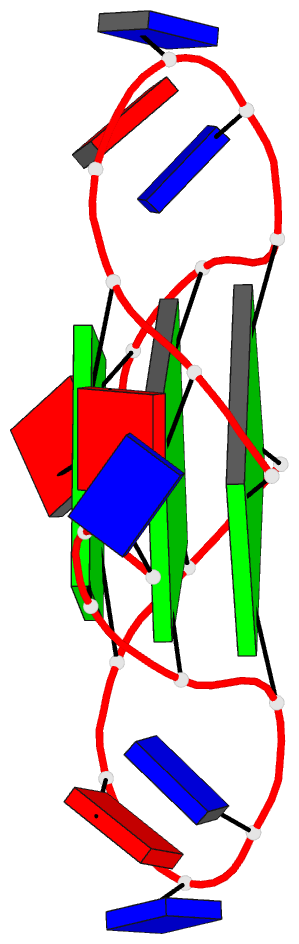
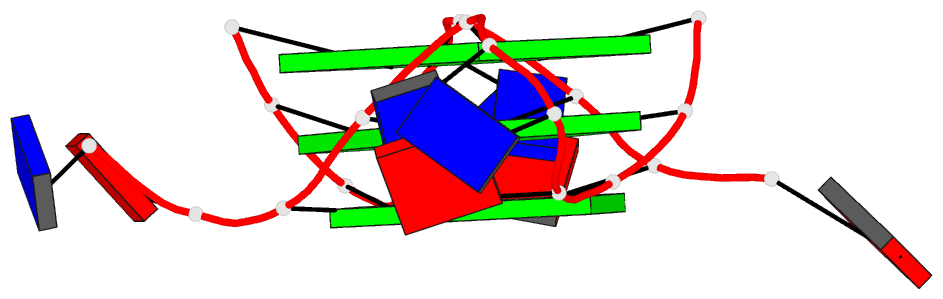
Summary information and primary citation
- PDB-id
-
6ip7;
SNAP-derived features in text and
JSON formats
- Class
- DNA
- Method
- X-ray (1.55 Å)
- Summary
- Structure of human telomeric DNA with
5-selenophene-modified deoxyuridine at residue 11
- Reference
-
Nuthanakanti A, Ahmed I, Khatik SY, Saikrishnan K,
Srivatsan SG (2019): "Probing
G-quadruplex topologies and recognition concurrently in
real time and 3D using a dual-app nucleoside probe."
Nucleic Acids Res., 47,
6059-6072. doi: 10.1093/nar/gkz419.
- Abstract
- Comprehensive understanding of structure and
recognition properties of regulatory nucleic acid elements
in real time and atomic level is highly important to devise
efficient therapeutic strategies. Here, we report the
establishment of an innovative biophysical platform using a
dual-app nucleoside analog, which serves as a common probe
to detect and correlate different GQ structures and ligand
binding under equilibrium conditions and in 3D by
fluorescence and X-ray crystallography techniques. The
probe (SedU) is composed of a microenvironment-sensitive
fluorophore and an excellent anomalous X-ray scatterer
(Se), which is assembled by attaching a selenophene ring at
5-position of 2'-deoxyuridine. SedU incorporated into the
loop region of human telomeric DNA repeat fluorescently
distinguished subtle differences in GQ topologies and
enabled quantify ligand binding to different topologies.
Importantly, anomalous X-ray dispersion signal from Se
could be used to determine the structure of GQs. As the
probe is minimally perturbing, a direct comparison of
fluorescence data and crystal structures provided
structural insights on how the probe senses different GQ
conformations without affecting the native fold. Taken
together, our dual-app probe represents a new class of tool
that opens up new experimental strategies to concurrently
investigate nucleic acid structure and recognition in real
time and 3D.