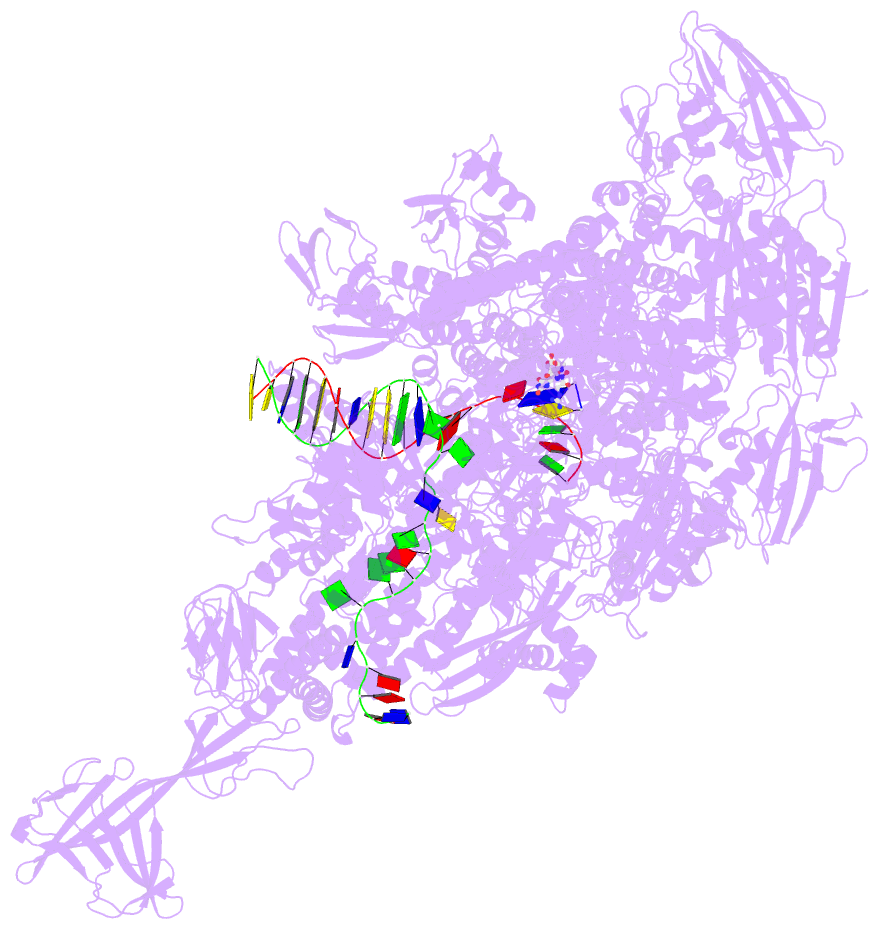
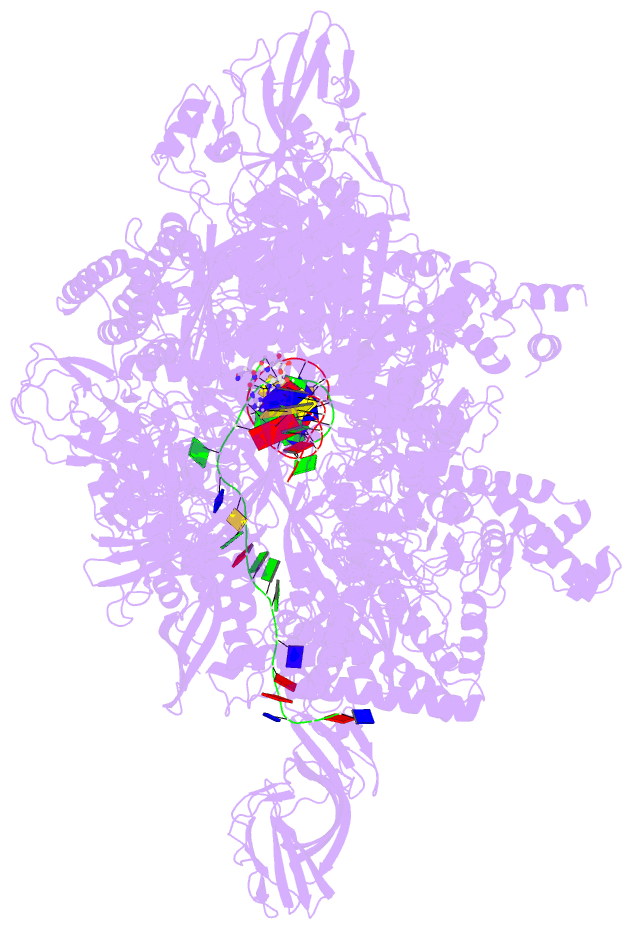
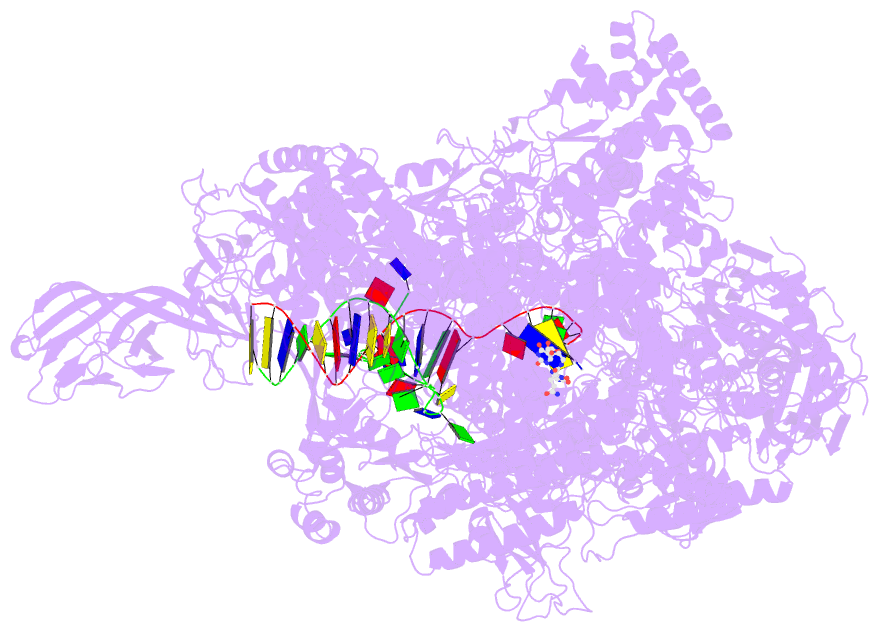
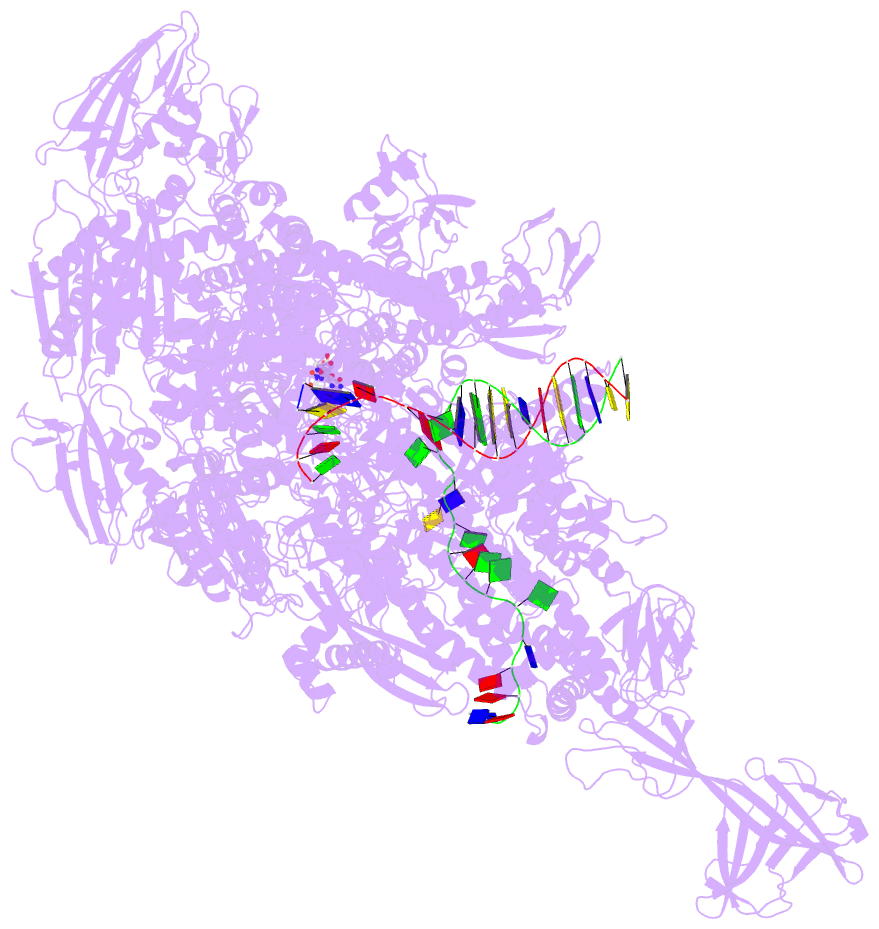
Summary information and primary citation
- PDB-id
- 5x21; DSSR-derived features in text and JSON formats
- Class
- transferase-DNA
- Method
- X-ray (3.323 Å)
- Summary
- Crystal structure of thermus thermophilus transcription initiation complex with gpa and pseudouridimycin (pum)
- Reference
- Maffioli SI, Zhang Y, Degen D, Carzaniga T, Del Gatto G, Serina S, Monciardini P, Mazzetti C, Guglierame P, Candiani G, Chiriac AI, Facchetti G, Kaltofen P, Sahl HG, Deho G, Donadio S, Ebright RH (2017): "Antibacterial Nucleoside-Analog Inhibitor of Bacterial RNA Polymerase." Cell, 169, 1240-1248.e23. doi: 10.1016/j.cell.2017.05.042.
- Abstract
- Drug-resistant bacterial pathogens pose an urgent public-health crisis. Here, we report the discovery, from microbial-extract screening, of a nucleoside-analog inhibitor that inhibits bacterial RNA polymerase (RNAP) and exhibits antibacterial activity against drug-resistant bacterial pathogens: pseudouridimycin (PUM). PUM is a natural product comprising a formamidinylated, N-hydroxylated Gly-Gln dipeptide conjugated to 6'-amino-pseudouridine. PUM potently and selectively inhibits bacterial RNAP in vitro, inhibits bacterial growth in culture, and clears infection in a mouse model of Streptococcus pyogenes peritonitis. PUM inhibits RNAP through a binding site on RNAP (the NTP addition site) and mechanism (competition with UTP for occupancy of the NTP addition site) that differ from those of the RNAP inhibitor and current antibacterial drug rifampin (Rif). PUM exhibits additive antibacterial activity when co-administered with Rif, exhibits no cross-resistance with Rif, and exhibits a spontaneous resistance rate an order-of-magnitude lower than that of Rif. PUM is a highly promising lead for antibacterial therapy.