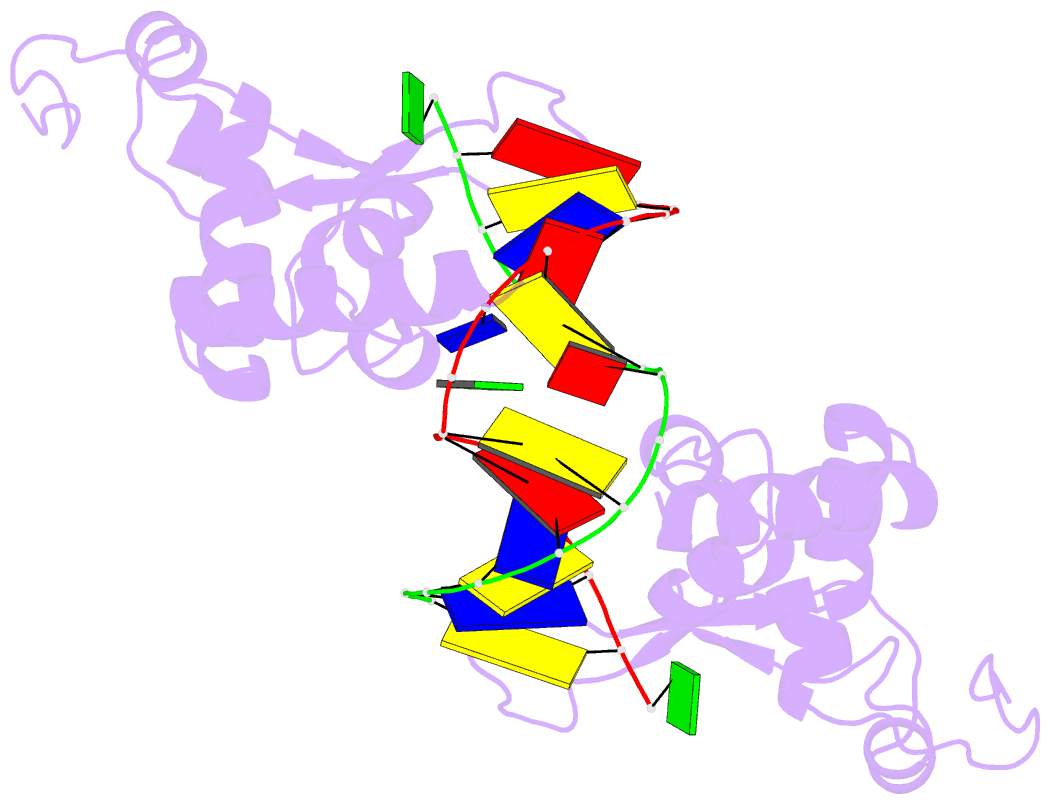
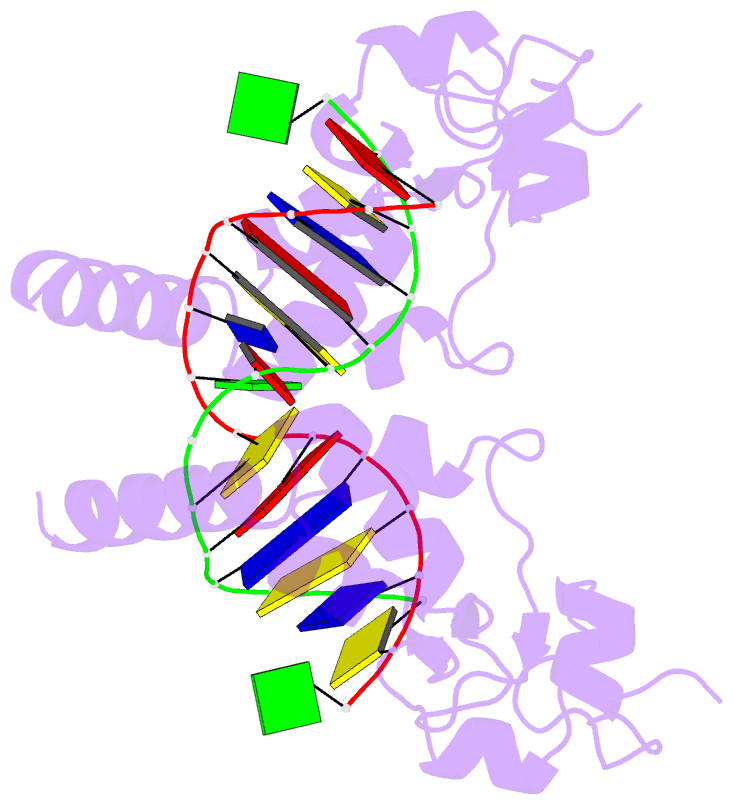
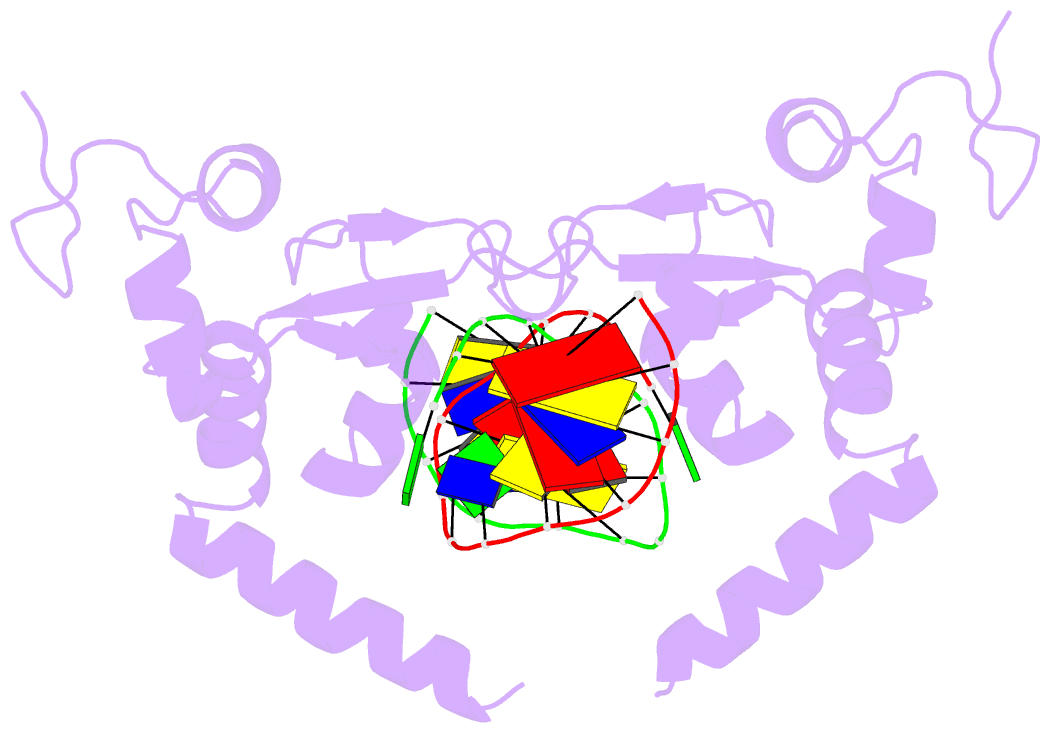
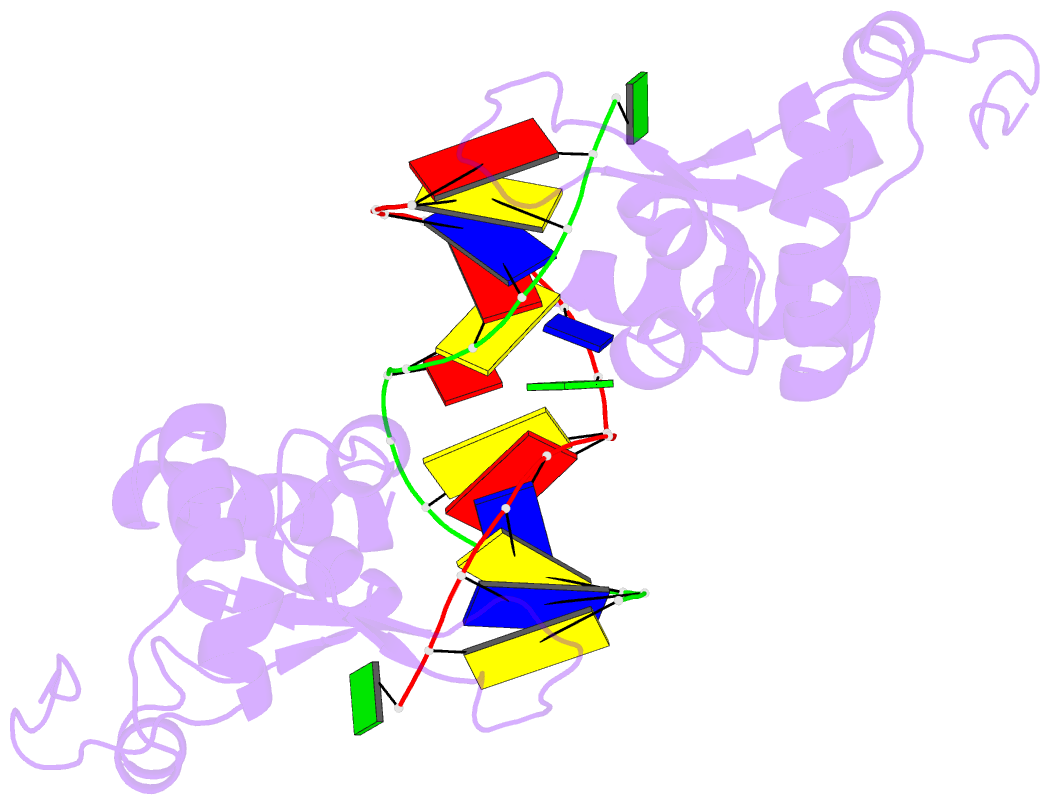
Summary information and primary citation
- PDB-id
- 5lcl; DSSR-derived features in text and JSON formats
- Class
- DNA binding protein
- Method
- X-ray (2.2 Å)
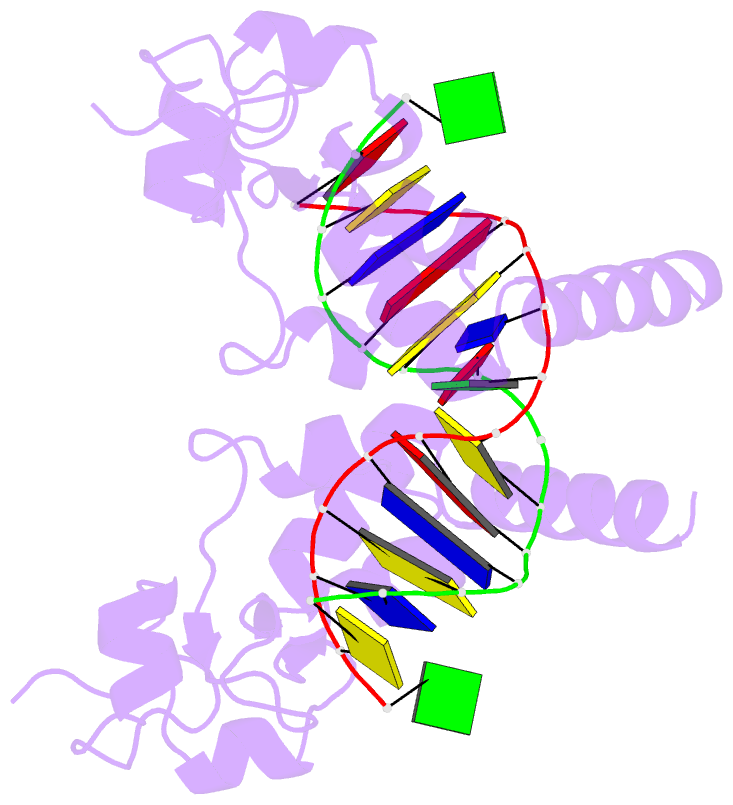
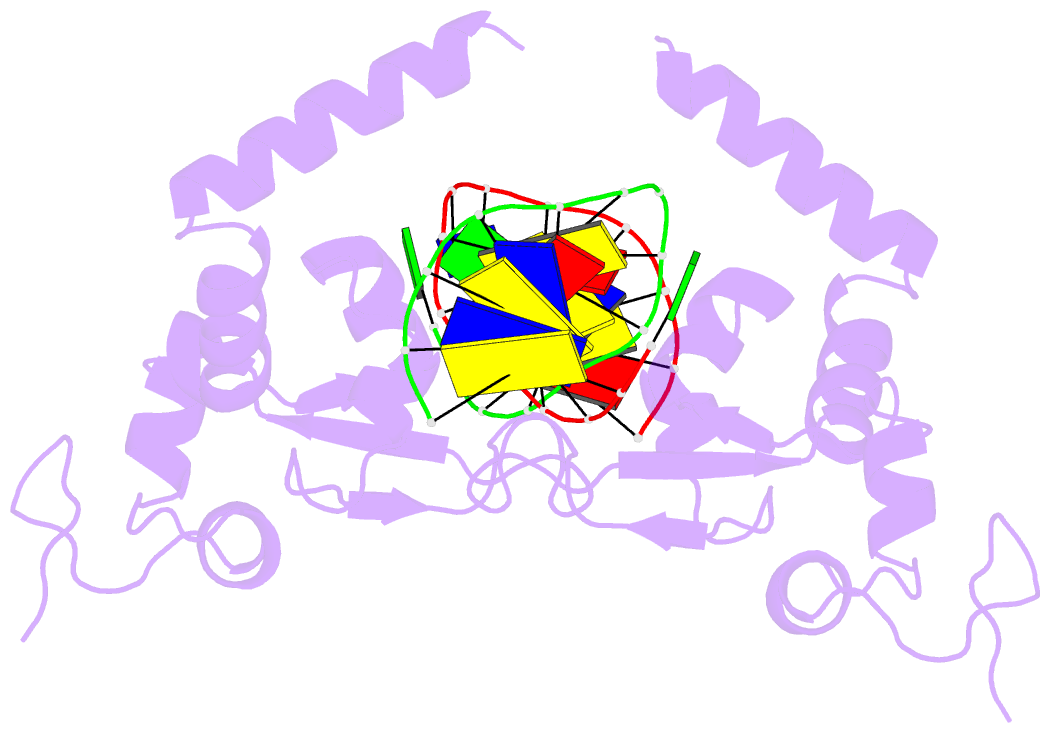
- Summary
- Structure of the rad14 DNA-binding domain in complex with c8-aminofluorene- guanine containing DNA
- Reference
- Ebert C, Simon N, Schneider S, Carell T (2017): "Structural Insights into the Recognition of N(2) -Aryl- and C8-Aryl DNA Lesions by the Repair Protein XPA/Rad14." Chembiochem, 18, 1379-1382. doi: 10.1002/cbic.201700169.
- Abstract
- Aromatic amines are strongly carcinogenic compounds. They are activated in the liver to give reactive nitrenium ions that are known to react with the nucleobases within the DNA duplex. The reaction occurs predominantly at the C8-position of the dG base, which gives C8-acetyl-aryl-, or C8-aryl-dG adducts in an electrophilic aromatic substitution reaction. Alternatively, reaction with the exocyclic 2-NH₂ group is observed. While the C8-adducts retain the base pairing properties, base pairing is strongly compromised in the case of the N²-adducts. Here we show crystal structures of two DNA lesions, N²-acetylnaphthyl-dG and C8-fluorenyl-dG within a DNA duplex recognized by the repair protein Rad14. The structures confirm that two molecules of the repair protein recognize the lesion by induction of a 72° or 78° kink at the site of the lesion. Importantly, the same overall kinked structure is induced by binding of the repair proteins although the lesions differ structurally, resulting in distinct stacking interactions of the lesions within the duplex. The result suggests that the repair protein XPA/Rad14 is a sensor that recognizes flexibility. The protein converts the information that structurally different lesions are present in the duplex into a unifying sharply kinked recognition motif.