Summary information and primary citation
- PDB-id
- 5k98; DSSR-derived features in text and JSON formats
- Class
- transcription-DNA
- Method
- X-ray (3.99 Å)
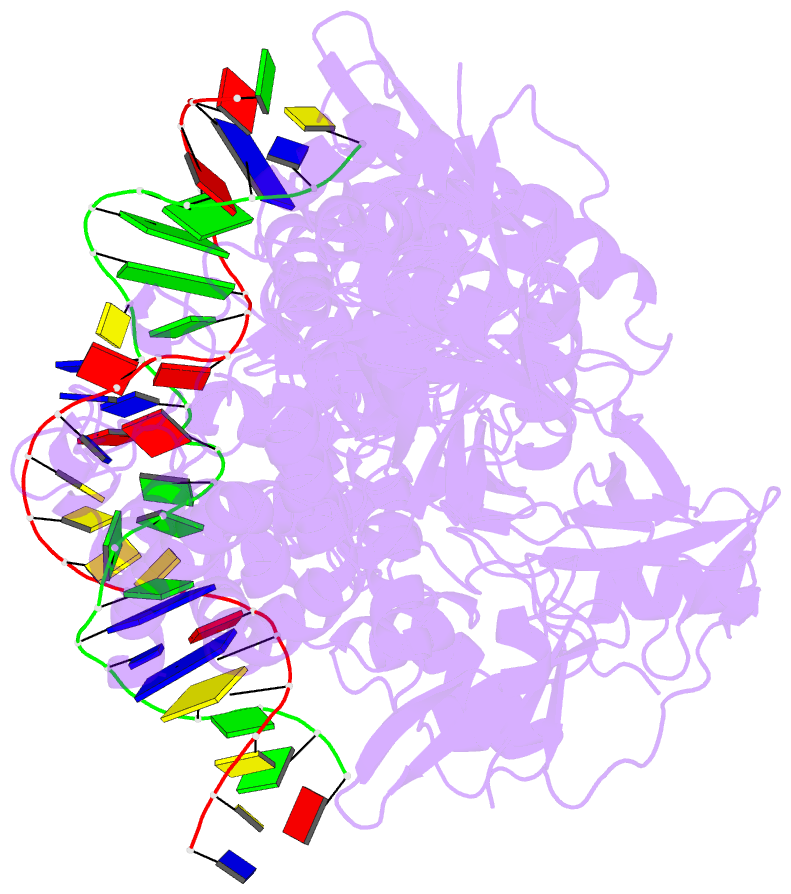
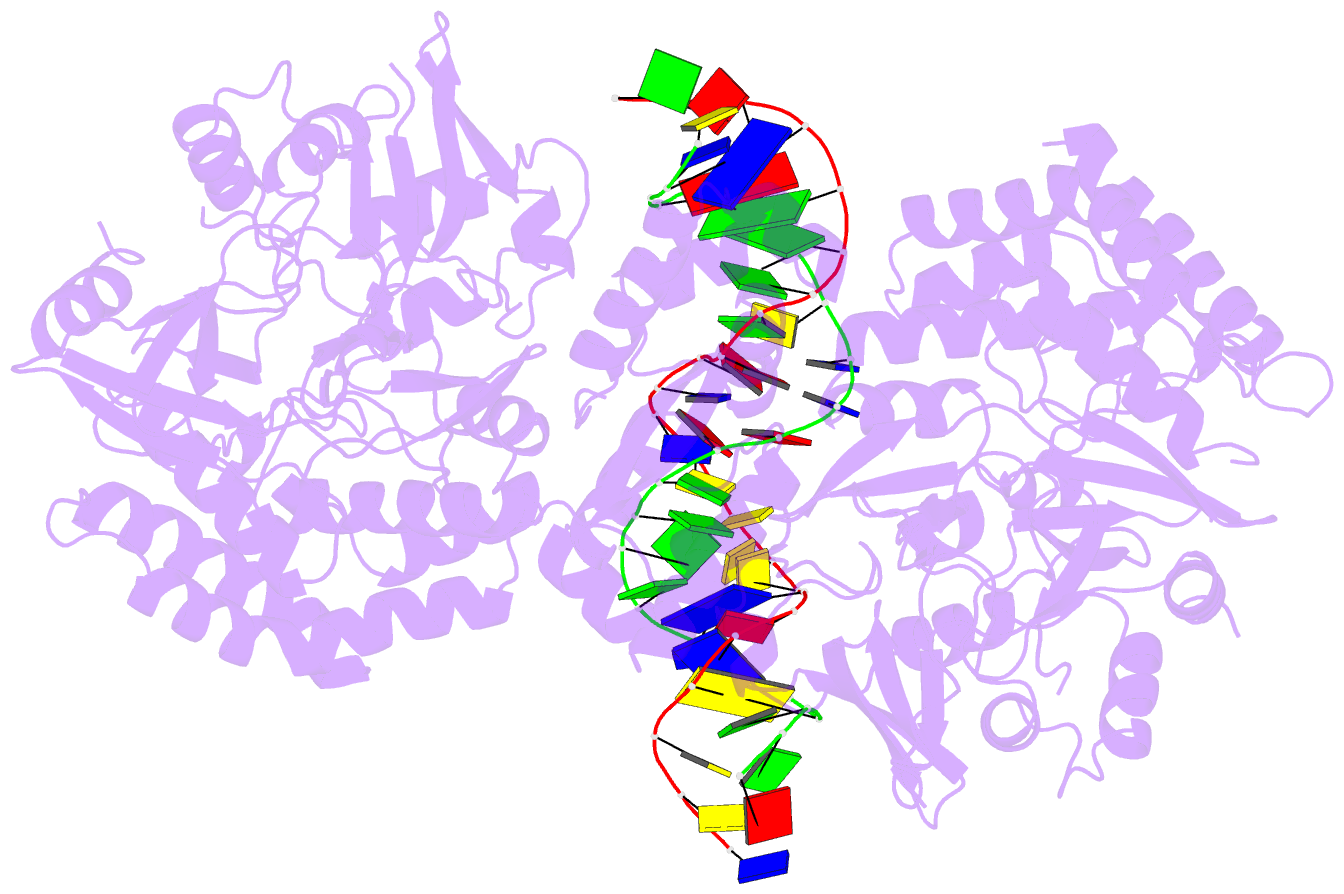
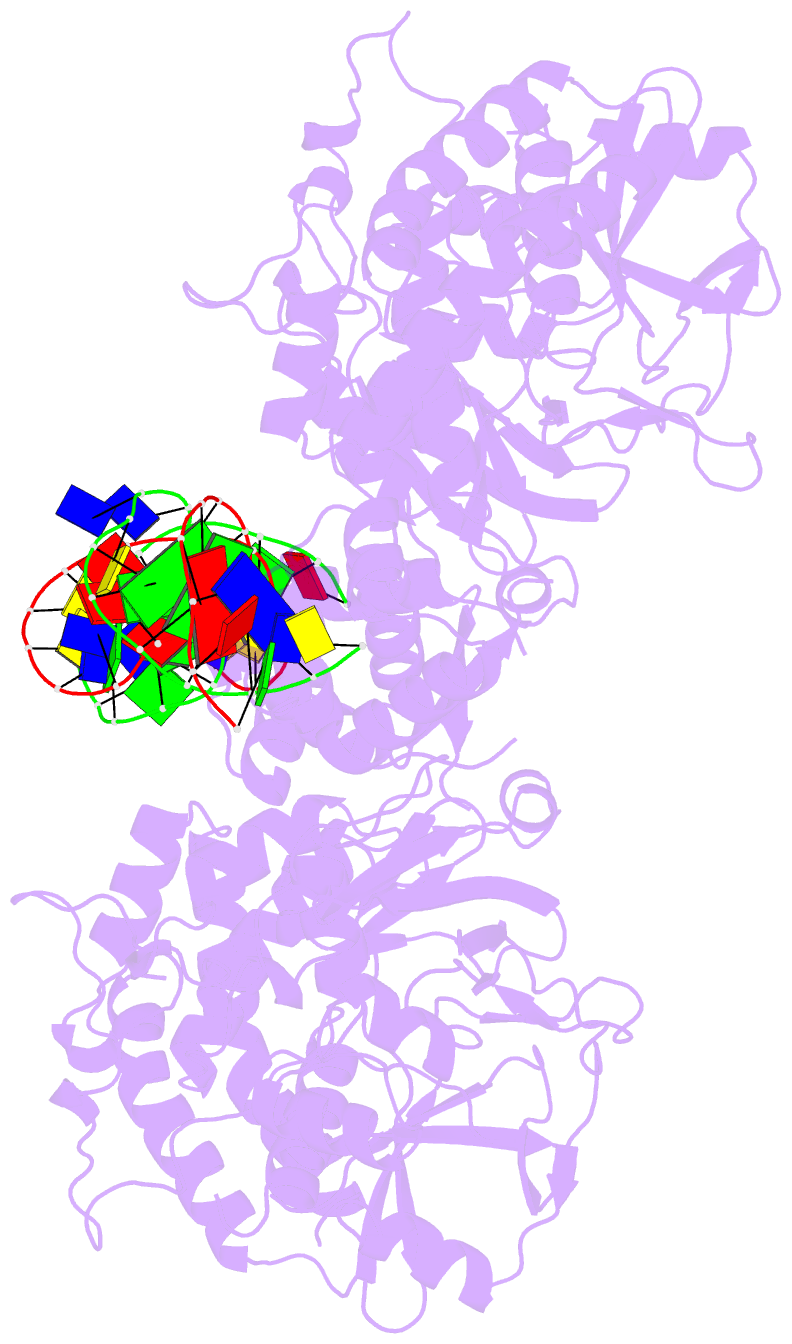
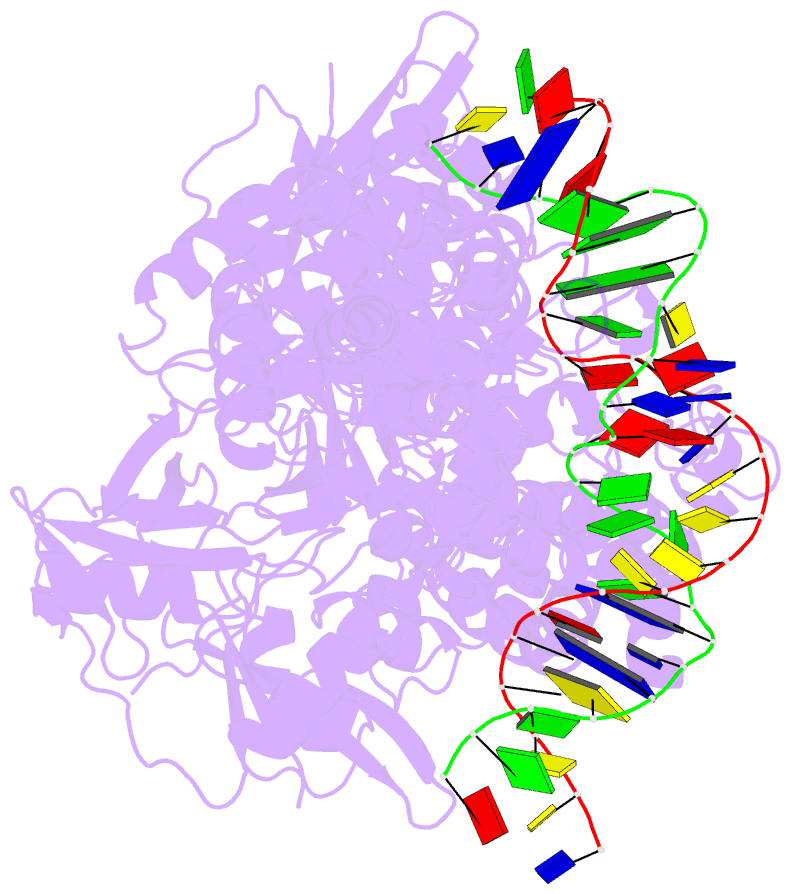
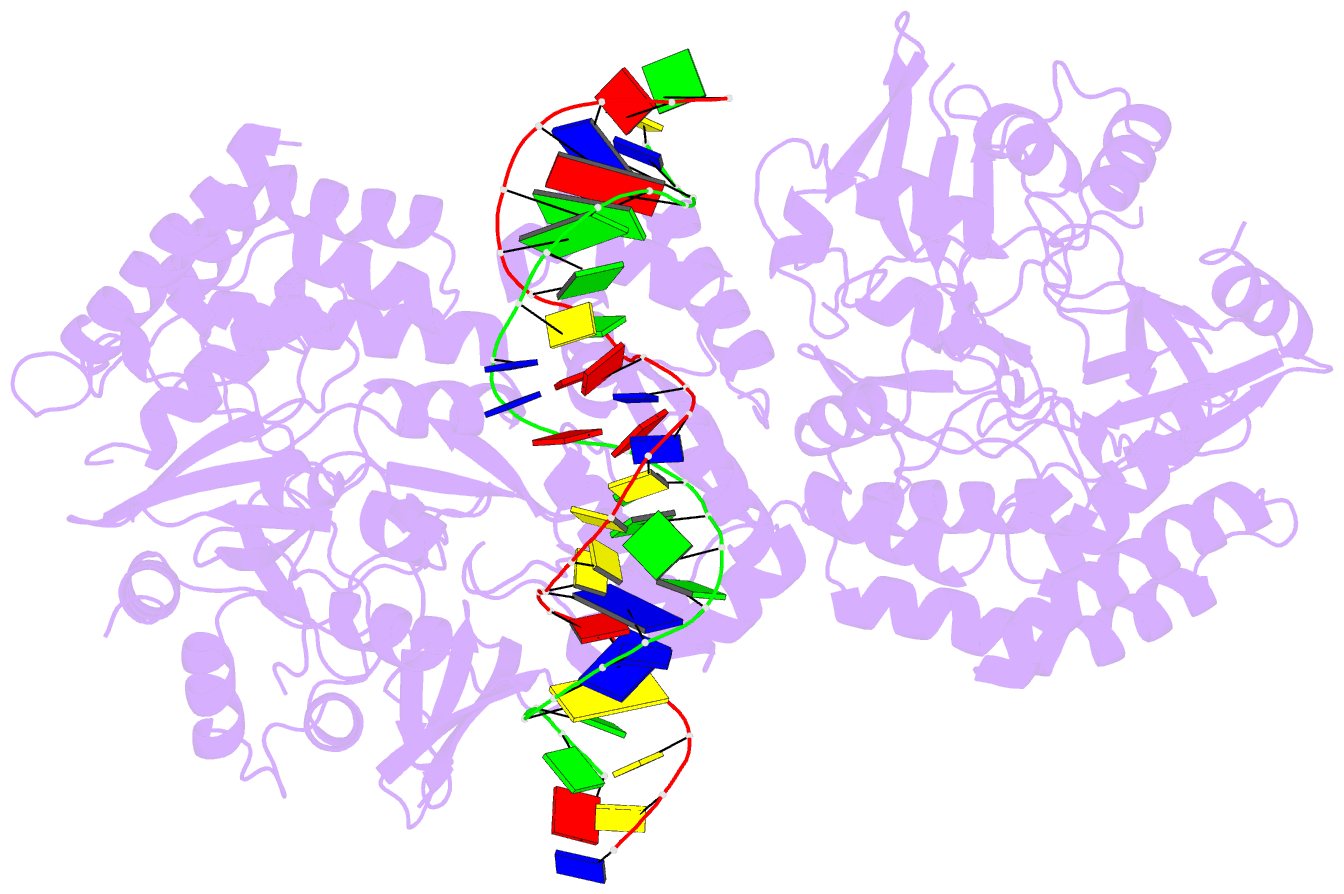
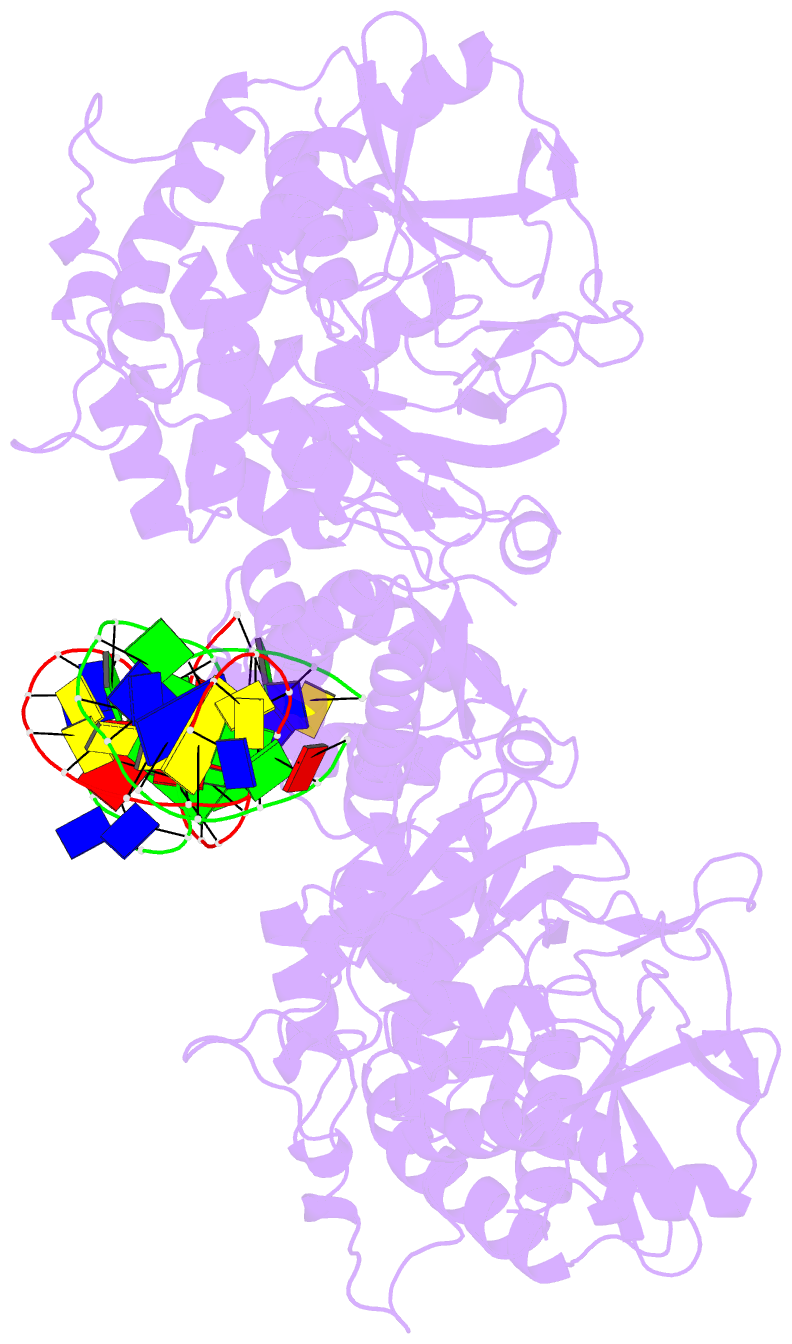
- Summary
- Structure of hipa-hipb-o2-o3 complex
- Reference
- Schumacher MA, Balani P, Min J, Chinnam NB, Hansen S, Vulic M, Lewis K, Brennan RG (2015): "HipBA-promoter structures reveal the basis of heritable multidrug tolerance." Nature, 524, 59-64. doi: 10.1038/nature14662.
- Abstract
- Multidrug tolerance is largely responsible for chronic infections and caused by a small population of dormant cells called persisters. Selection for survival in the presence of antibiotics produced the first genetic link to multidrug tolerance: a mutant in the Escherichia coli hipA locus. HipA encodes a serine-protein kinase, the multidrug tolerance activity of which is neutralized by binding to the transcriptional regulator HipB and hipBA promoter. The physiological role of HipA in multidrug tolerance, however, has been unclear. Here we show that wild-type HipA contributes to persister formation and that high-persister hipA mutants cause multidrug tolerance in urinary tract infections. Perplexingly, high-persister mutations map to the N-subdomain-1 of HipA far from its active site. Structures of higher-order HipA-HipB-promoter complexes reveal HipA forms dimers in these assemblies via N-subdomain-1 interactions that occlude their active sites. High-persistence mutations, therefore, diminish HipA-HipA dimerization, thereby unleashing HipA to effect multidrug tolerance. Thus, our studies reveal the mechanistic basis of heritable, clinically relevant antibiotic tolerance.