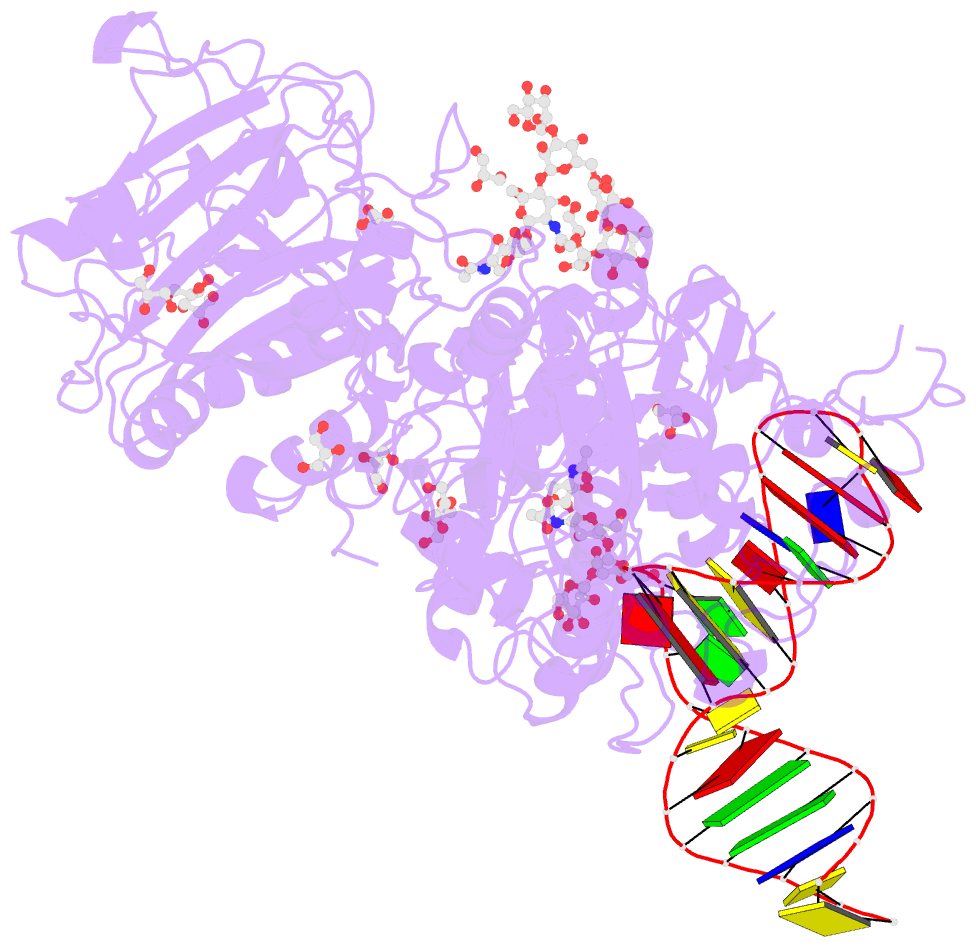
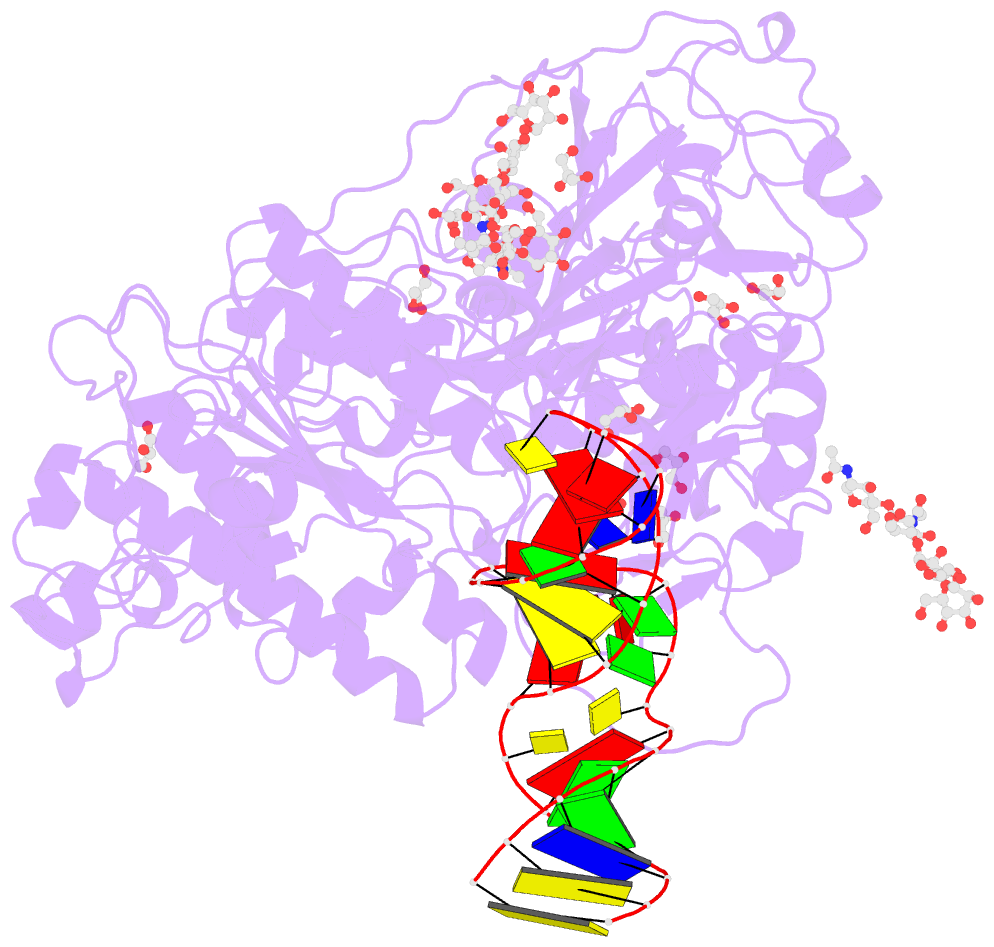
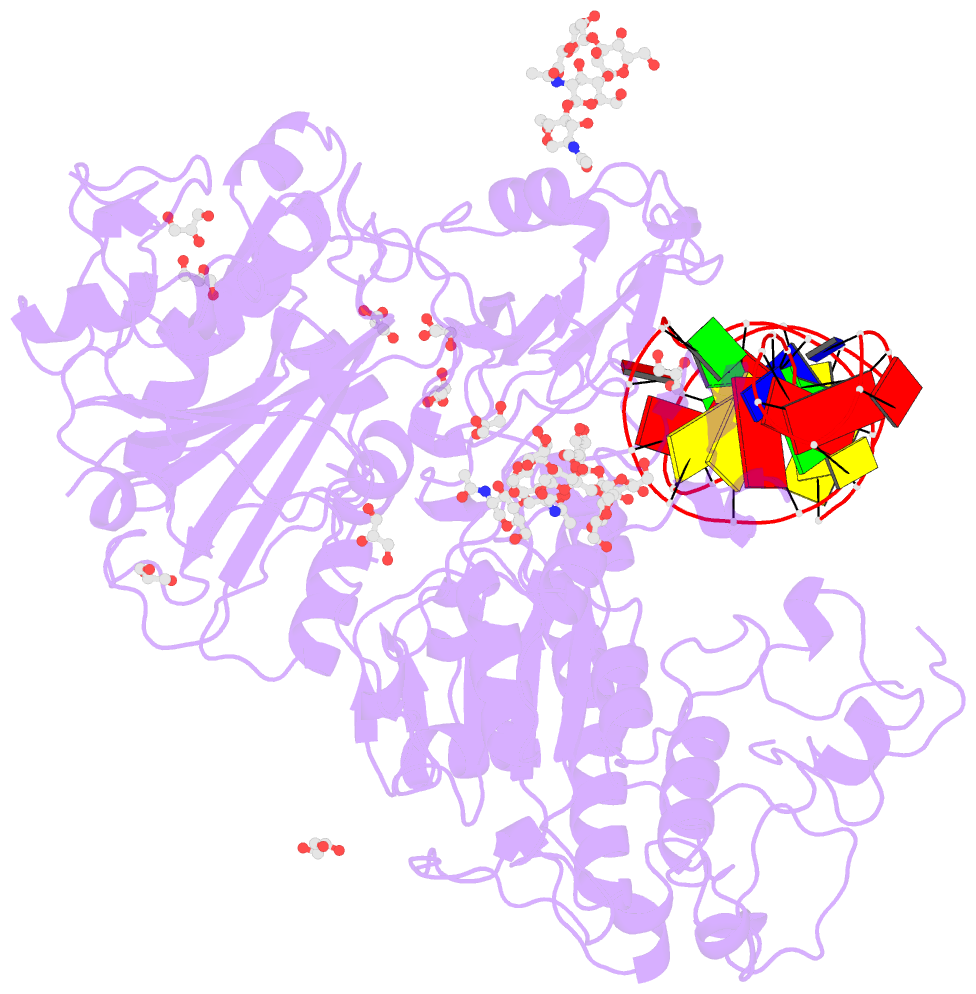
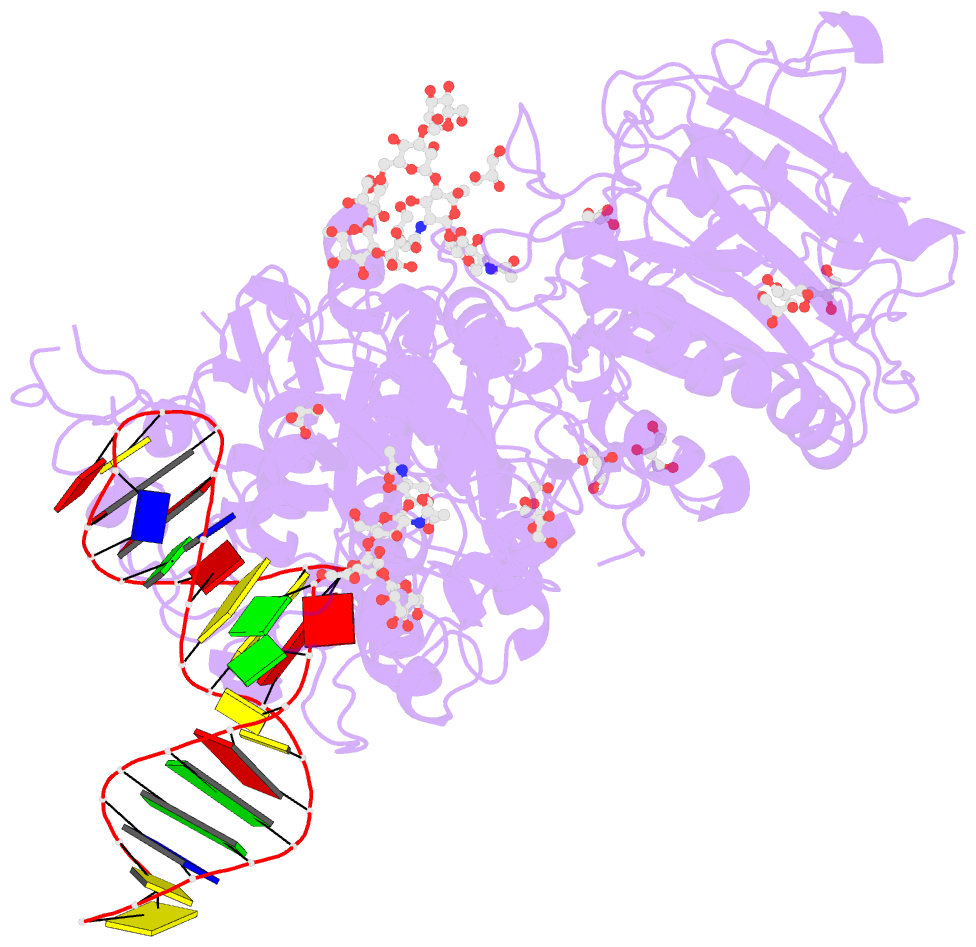
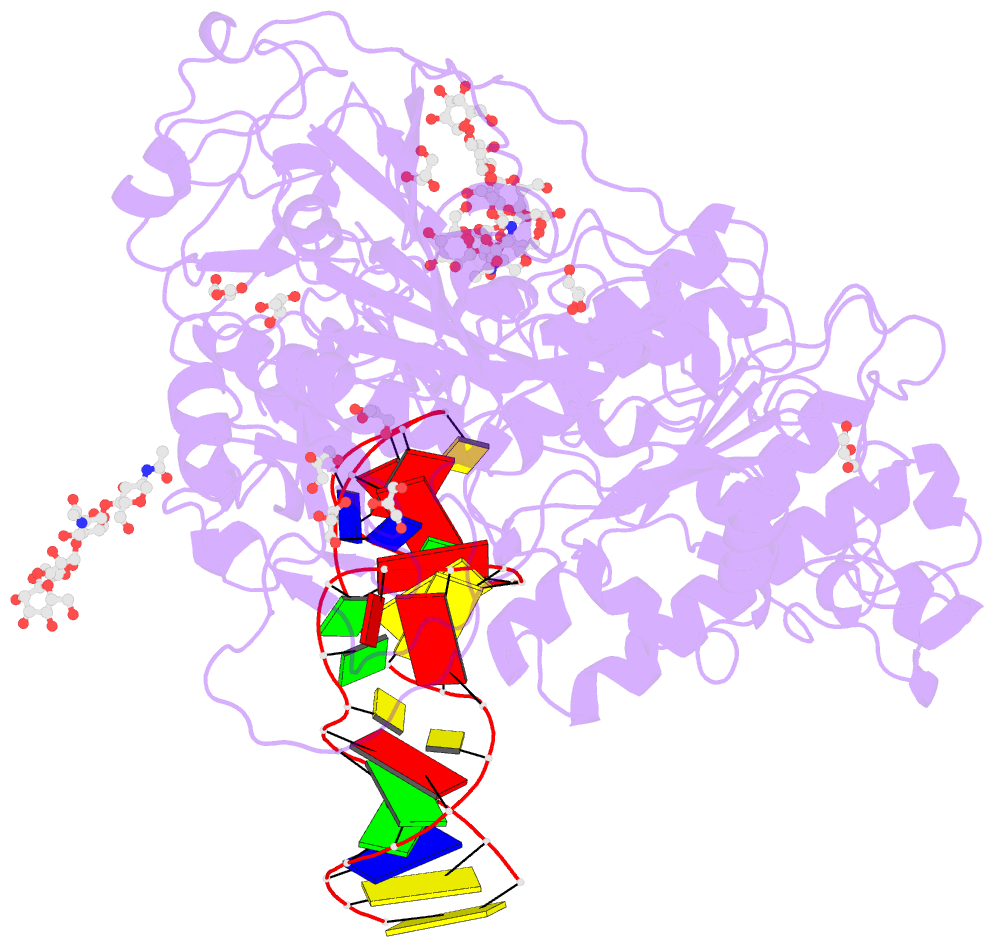
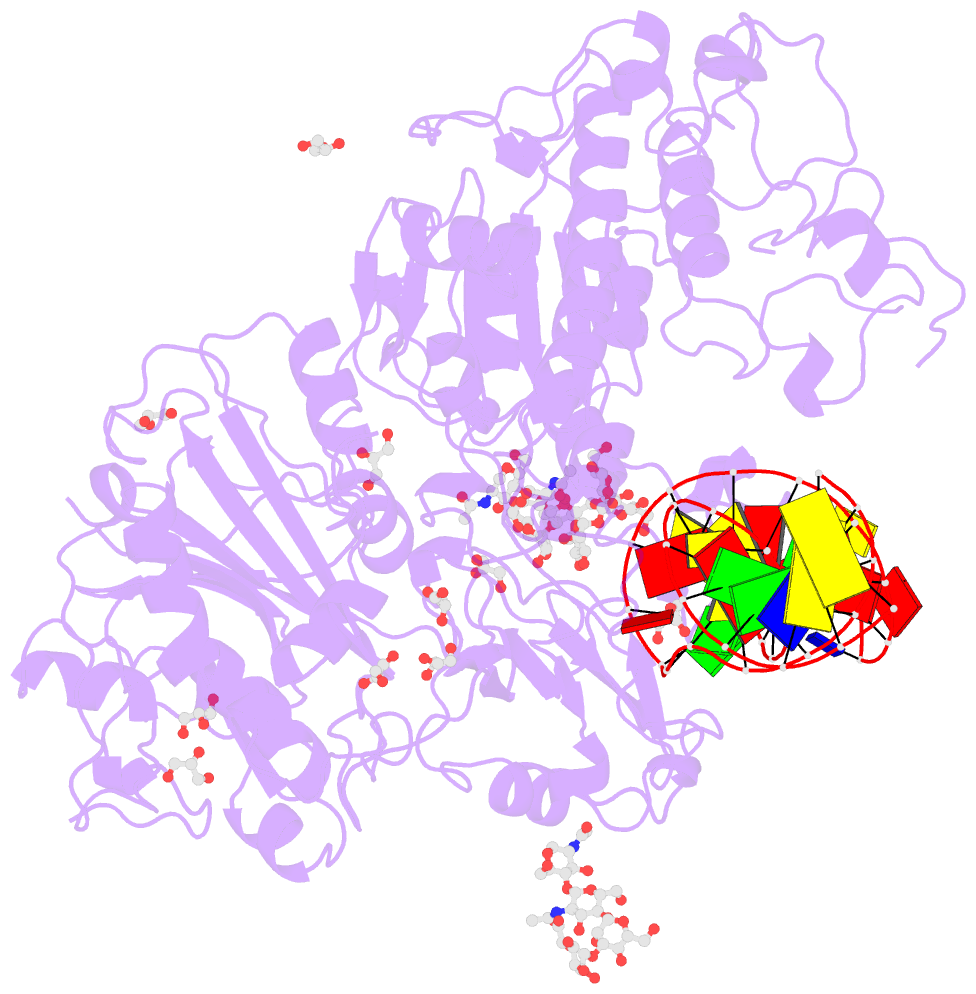
Summary information and primary citation
- PDB-id
- 5hrt; DSSR-derived features in text and JSON formats
- Class
- hydrolase
- Method
- X-ray (1.997 Å)
- Summary
- Crystal structure of mouse autotaxin in complex with a DNA aptamer
- Reference
- Kato K, Ikeda H, Miyakawa S, Futakawa S, Nonaka Y, Fujiwara M, Okudaira S, Kano K, Aoki J, Morita J, Ishitani R, Nishimasu H, Nakamura Y, Nureki O (2016): "Structural basis for specific inhibition of Autotaxin by a DNA aptamer." Nat.Struct.Mol.Biol., 23, 395-401. doi: 10.1038/nsmb.3200.
- Abstract
- ATX is a plasma lysophospholipase D that hydrolyzes lysophosphatidylcholine (LPC) and produces lysophosphatidic acid. To date, no ATX-inhibition-mediated treatment strategies for human diseases have been established. Here, we report anti-ATX DNA aptamers that inhibit ATX with high specificity and efficacy. We solved the crystal structure of ATX in complex with the anti-ATX aptamer RB011, at 2.0-Å resolution. RB011 binds in the vicinity of the active site through base-specific interactions, thus preventing the access of the choline moiety of LPC substrates. Using the structural information, we developed the modified anti-ATX DNA aptamer RB014, which exhibited in vivo efficacy in a bleomycin-induced pulmonary fibrosis mouse model. Our findings reveal the structural basis for the specific inhibition of ATX by the anti-ATX aptamer and highlight the therapeutic potential of anti-ATX aptamers for the treatment of human diseases, such as pulmonary fibrosis.