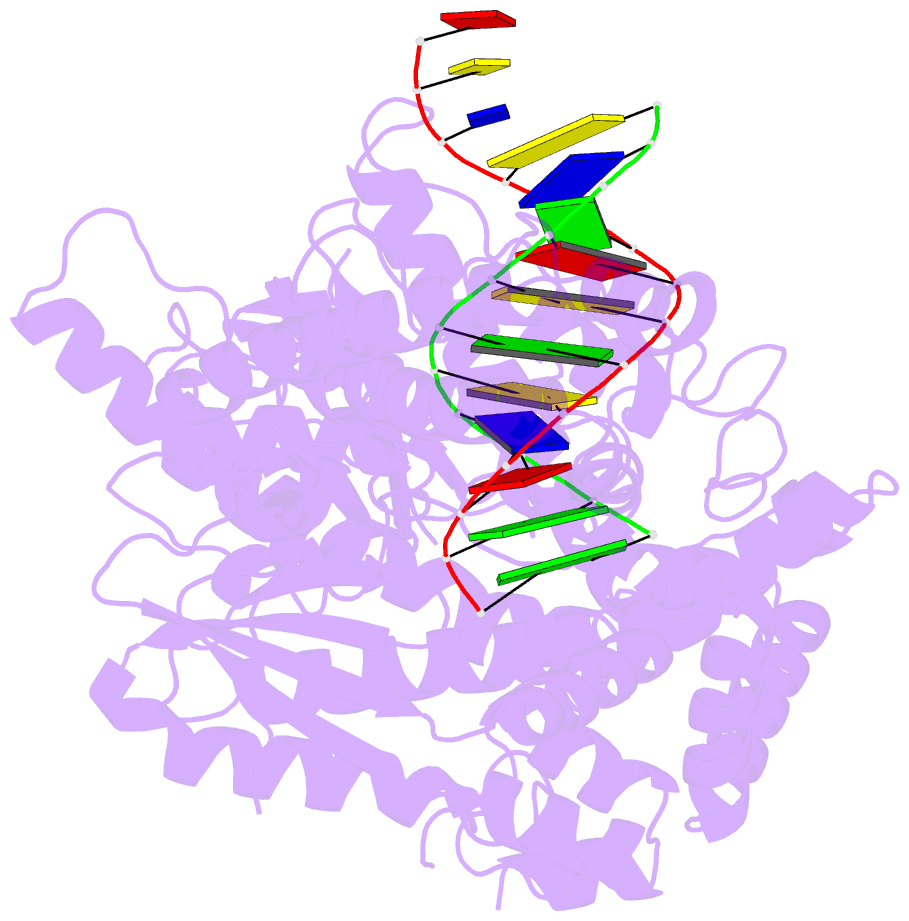
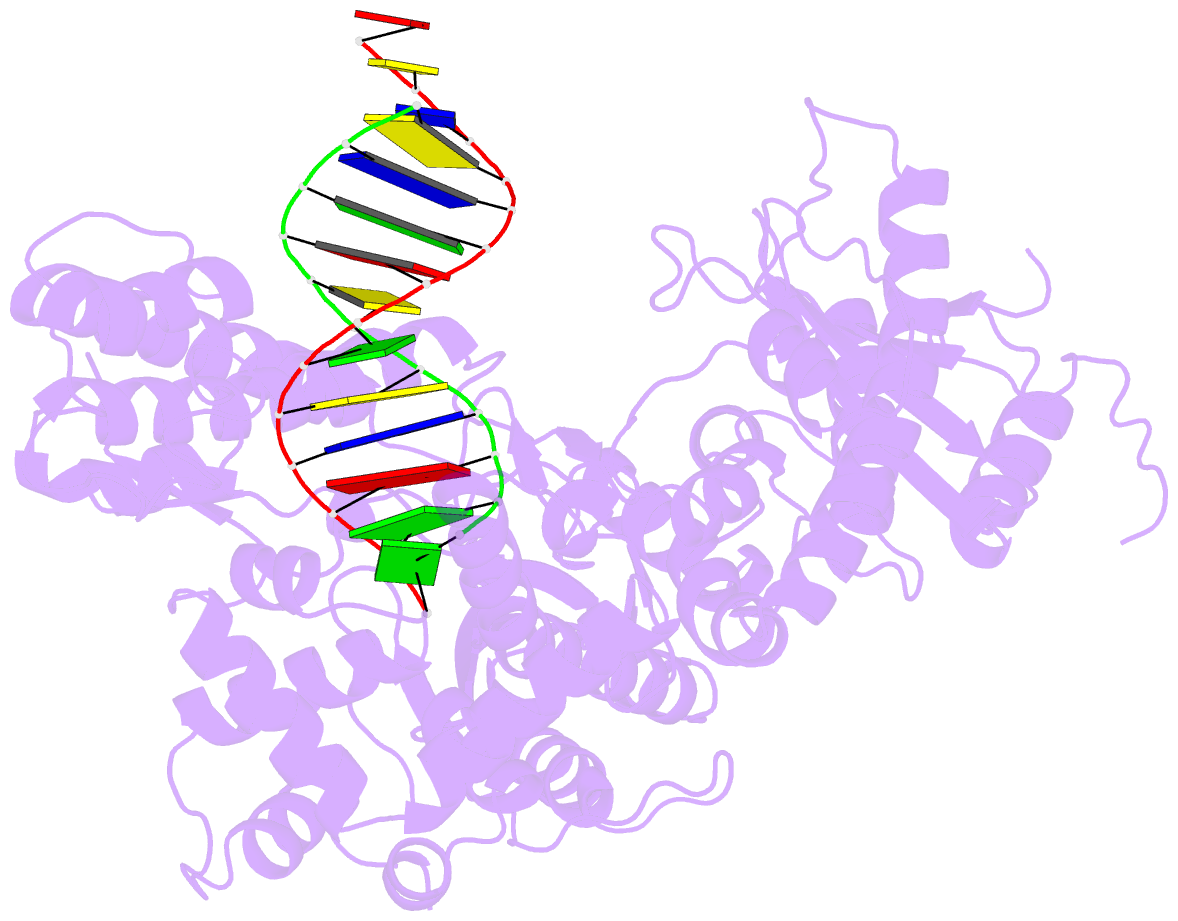
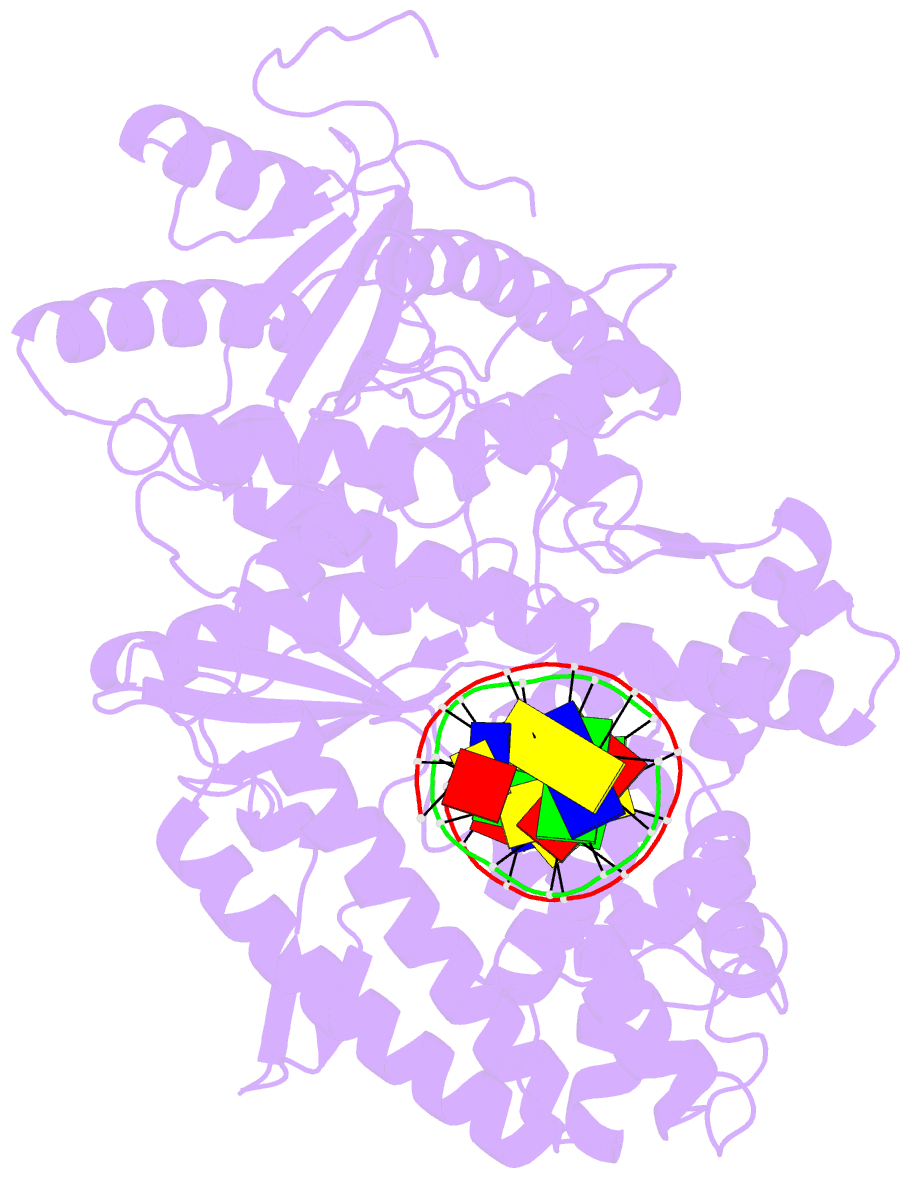
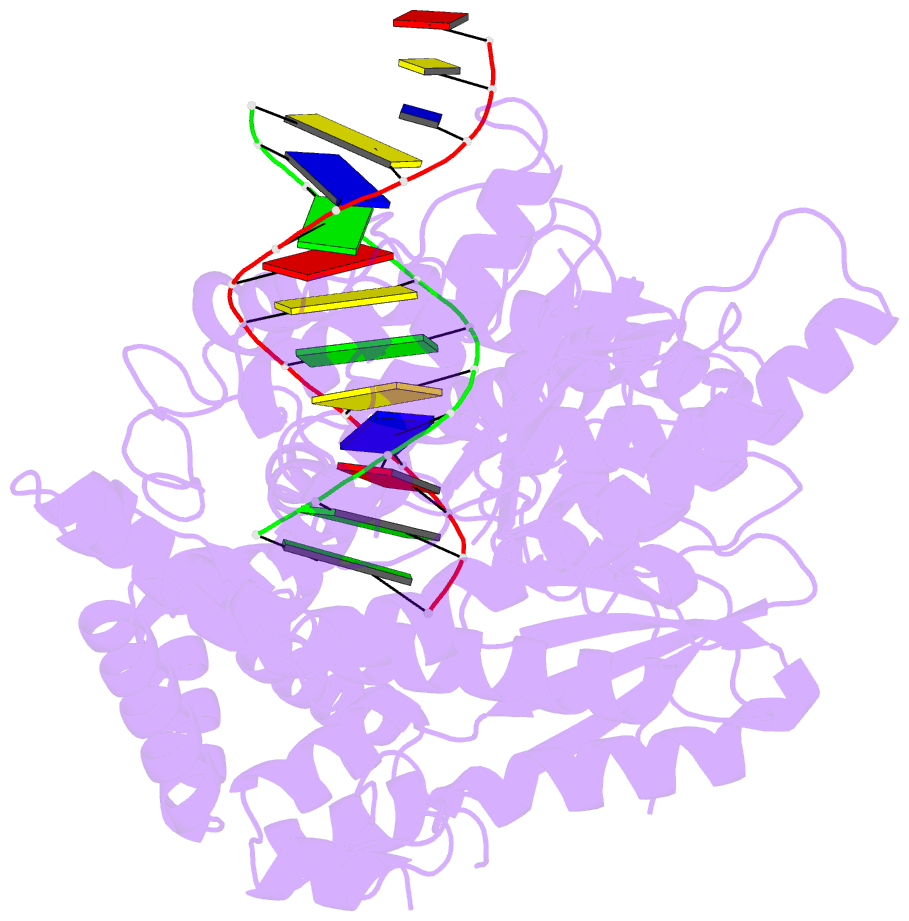
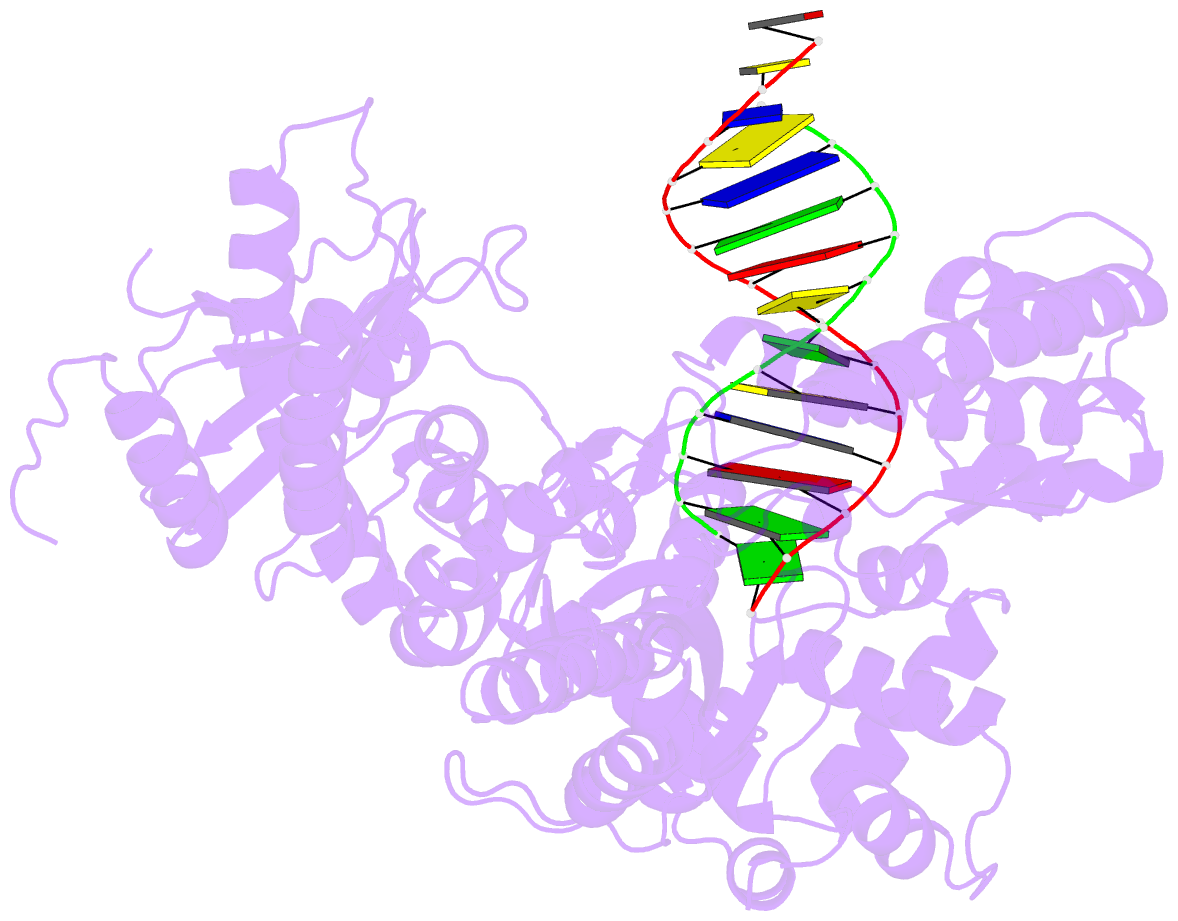
Summary information and primary citation
- PDB-id
- 4xvi; DSSR-derived features in text and JSON formats
- Class
- transferase-DNA
- Method
- X-ray (3.1 Å)
- Summary
- Binary complex of human polymerase nu and DNA with the finger domain ajar
- Reference
- Lee YS, Gao Y, Yang W (2015): "How a homolog of high-fidelity replicases conducts mutagenic DNA synthesis." Nat.Struct.Mol.Biol., 22, 298-303. doi: 10.1038/nsmb.2985.
- Abstract
- All DNA replicases achieve high fidelity by a conserved mechanism, but each translesion polymerase carries out mutagenic DNA synthesis in its own way. Here we report crystal structures of human DNA polymerase ν (Pol ν), which is homologous to high-fidelity replicases yet is error prone. Instead of a simple open-to-closed movement of the O helix upon binding of a correct incoming nucleotide, Pol ν has a different open state and requires the finger domain to swing sideways and undergo both opening and closing motions to accommodate the nascent base pair. A single-amino acid substitution in the O helix of the finger domain improves the fidelity of Pol ν nearly ten-fold. A unique cavity and the flexibility of the thumb domain allow Pol ν to generate and accommodate a looped-out primer strand. Primer loop-out may be a mechanism for DNA trinucloetide-repeat expansion.