Summary information and primary citation
- PDB-id
- 4x0p; DSSR-derived features in text and JSON formats
- Class
- transferase-DNA
- Method
- X-ray (3.911 Å)
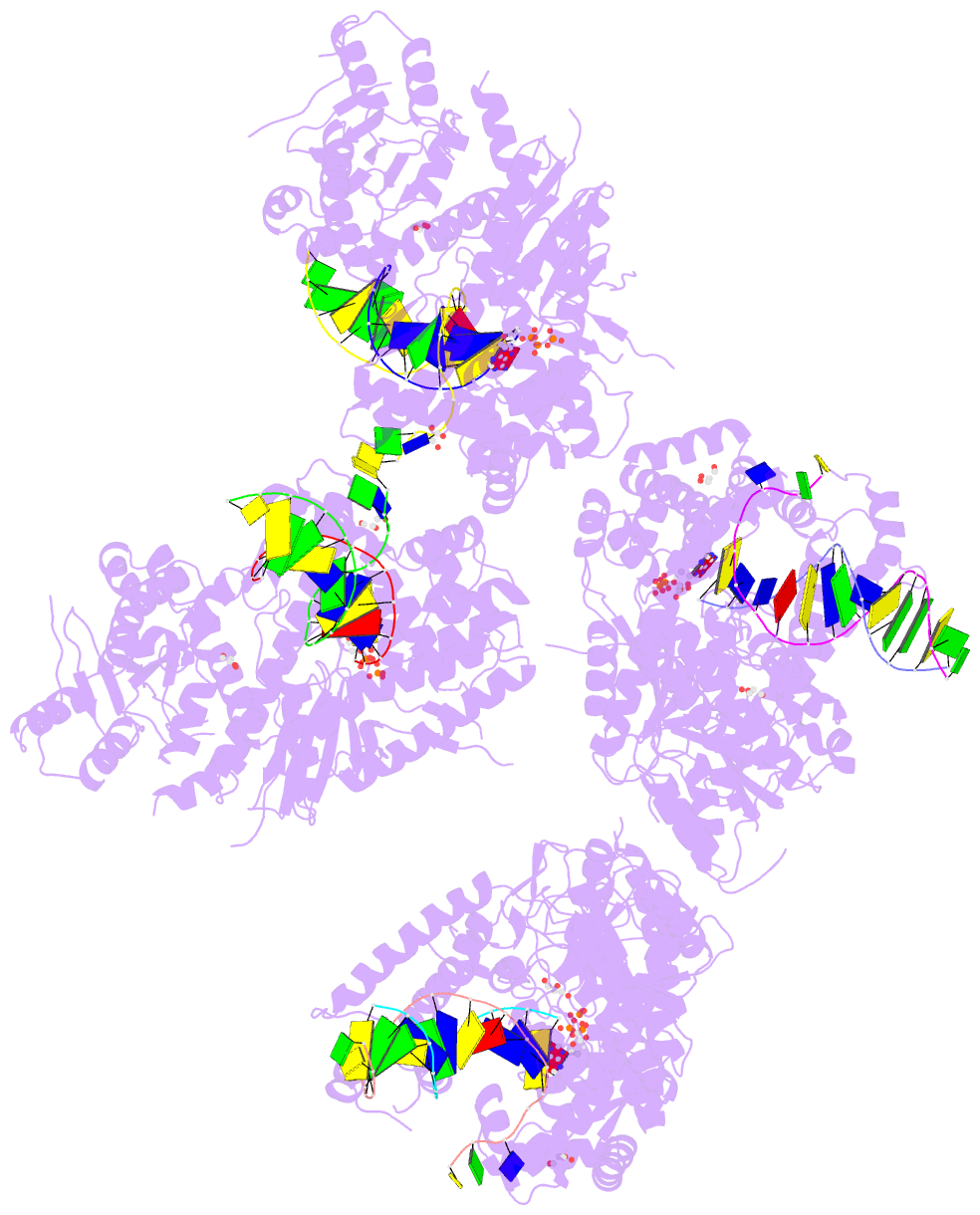
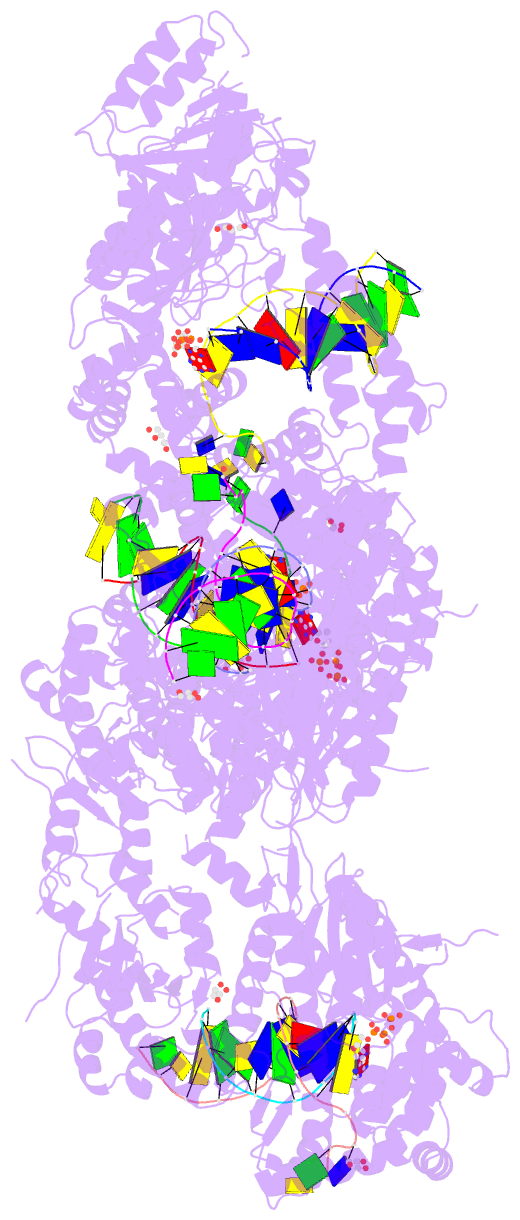
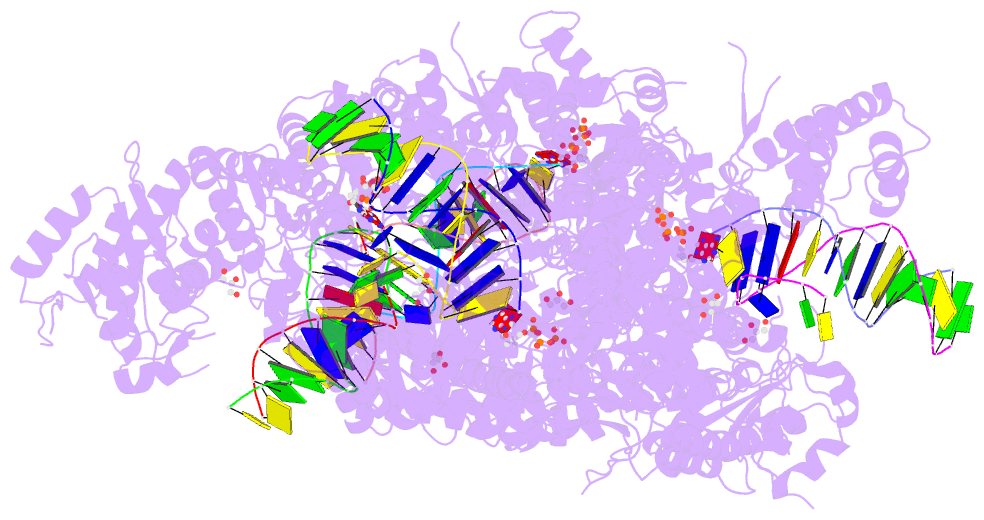
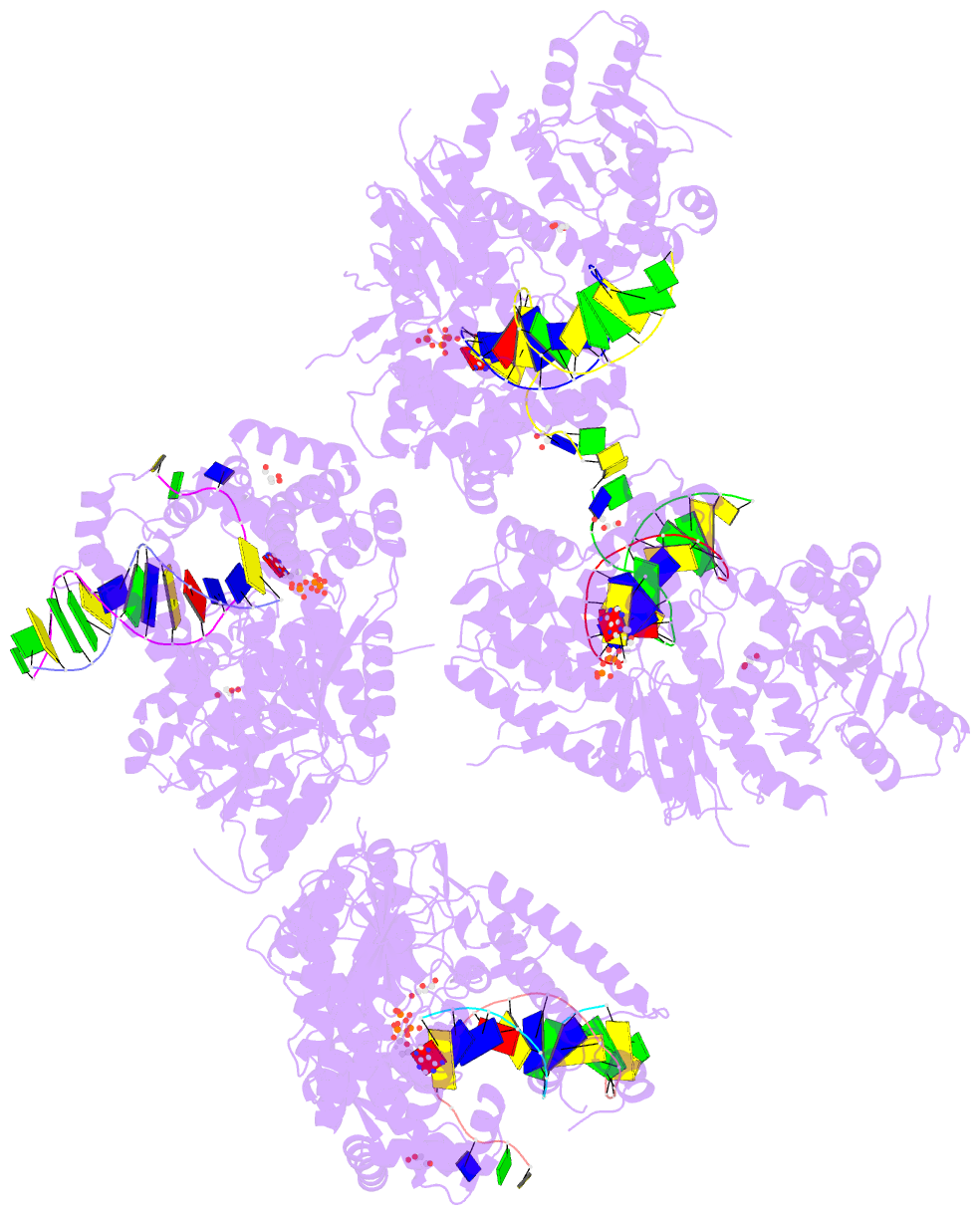
- Summary
- Ternary complex of human DNA polymerase theta c-terminal domain binding ddatp opposite a tetrahydrofuran ap site analog
- Reference
- Zahn KE, Averill AM, Aller P, Wood RD, Doublie S (2015): "Human DNA polymerase theta grasps the primer terminus to mediate DNA repair." Nat.Struct.Mol.Biol., 22, 304-311. doi: 10.1038/nsmb.2993.
- Abstract
- DNA polymerase θ protects against genomic instability via an alternative end-joining repair pathway for DNA double-strand breaks. Polymerase θ is overexpressed in breast, lung and oral cancers, and reduction of its activity in mammalian cells increases sensitivity to double-strand break-inducing agents, including ionizing radiation. Reported here are crystal structures of the C-terminal polymerase domain from human polymerase θ, illustrating two potential modes of dimerization. One structure depicts insertion of ddATP opposite an abasic-site analog during translesion DNA synthesis. The second structure describes a cognate ddGTP complex. Polymerase θ uses a specialized thumb subdomain to establish unique upstream contacts to the primer DNA strand, including an interaction with the 3'-terminal phosphate from one of five distinctive insertion loops. These observations demonstrate how polymerase θ grasps the primer to bypass DNA lesions or extend poorly annealed DNA termini to mediate end-joining.