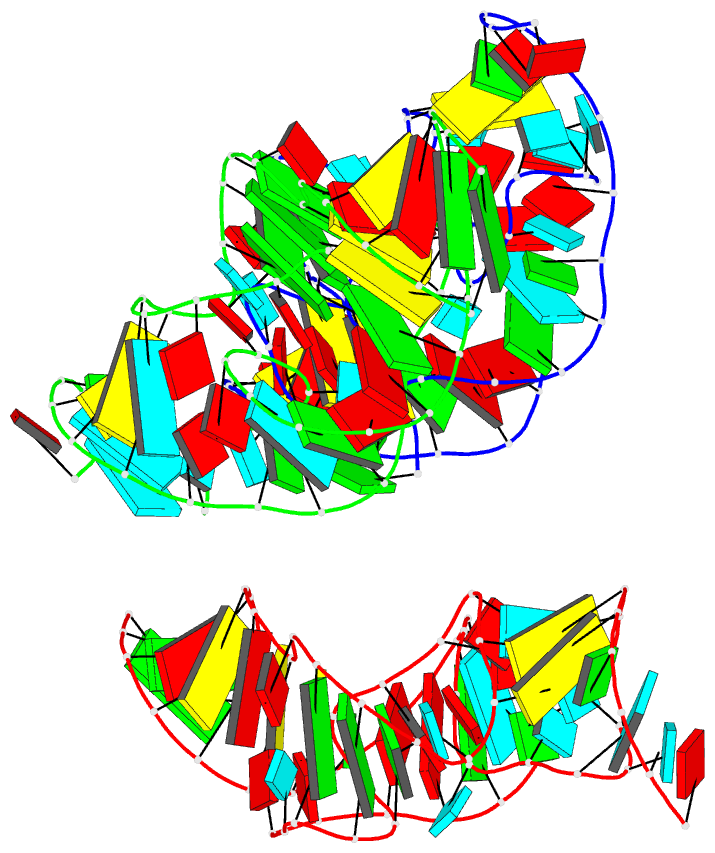
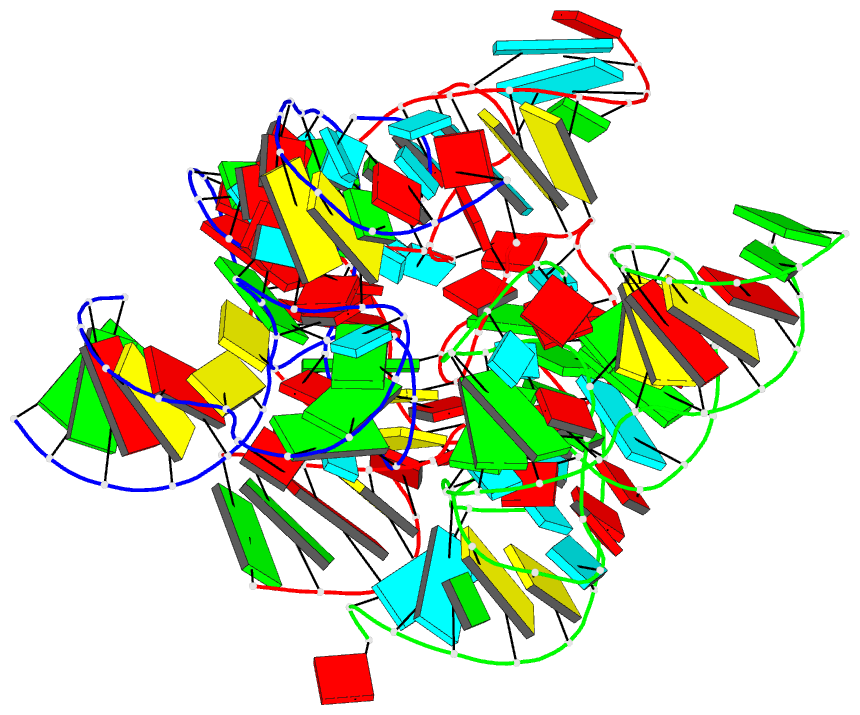
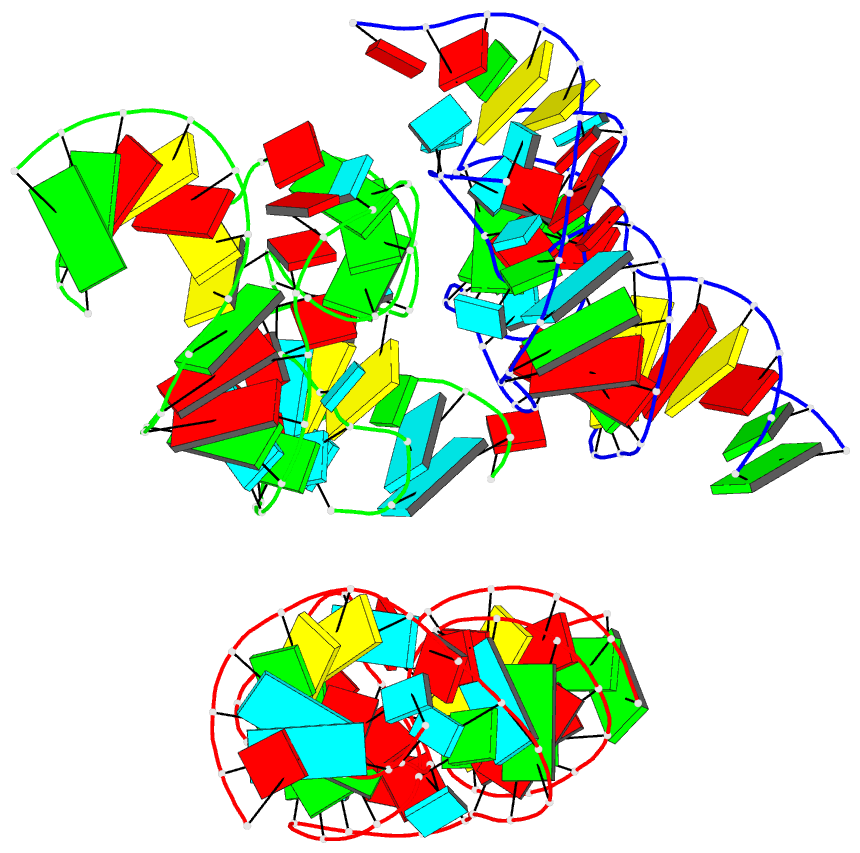
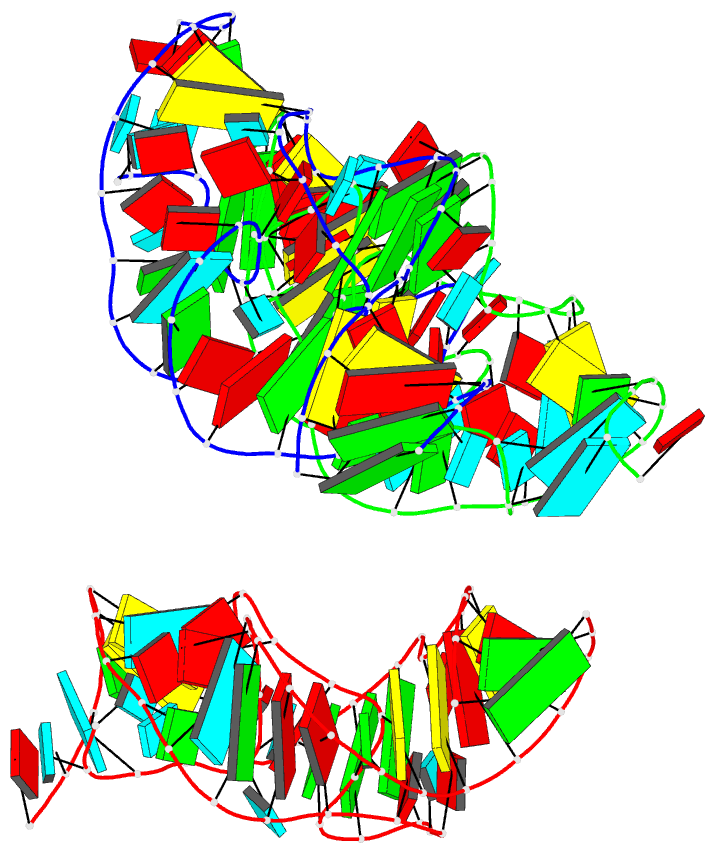
Summary information and primary citation
- PDB-id
-
4rgf;
SNAP-derived features in text and
JSON formats
- Class
- RNA
- Method
- X-ray (3.2 Å)
- Summary
- Crystal structure of the in-line aligned env22 twister
ribozyme soaked with mn2+
- Reference
-
Ren A, Kosutic M, Rajashankar KR, Frener M, Santner T,
Westhof E, Micura R, Patel DJ (2014): "In-line
alignment and Mg(2+) coordination at the cleavage site of
the env22 twister ribozyme." Nat Commun,
5, 5534. doi: 10.1038/ncomms6534.
- Abstract
- Small self-cleaving nucleolytic ribozymes contain
catalytic domains that accelerate site-specific
cleavage/ligation of phosphodiester backbones. We report on
the 2.9-Å crystal structure of the env22 twister ribozyme,
which adopts a compact tertiary fold stabilized by
co-helical stacking, double-pseudoknot formation and
long-range pairing interactions. The U-A cleavage site
adopts a splayed-apart conformation with the modelled 2'-O
of U positioned for in-line attack on the adjacent
to-be-cleaved P-O5' bond. Both an invariant guanosine and a
Mg(2+) are directly coordinated to the non-bridging
phosphate oxygens at the U-A cleavage step, with the former
positioned to contribute to catalysis and the latter to
structural integrity. The impact of key mutations on
cleavage activity identified an invariant guanosine that
contributes to catalysis. Our structure of the in-line
aligned env22 twister ribozyme is compared with two
recently reported twister ribozymes structures, which adopt
similar global folds, but differ in conformational features
around the cleavage site.