Summary information and primary citation
- PDB-id
- 4qgu; DSSR-derived features in text and JSON formats
- Class
- transcription-DNA
- Method
- X-ray (2.545 Å)
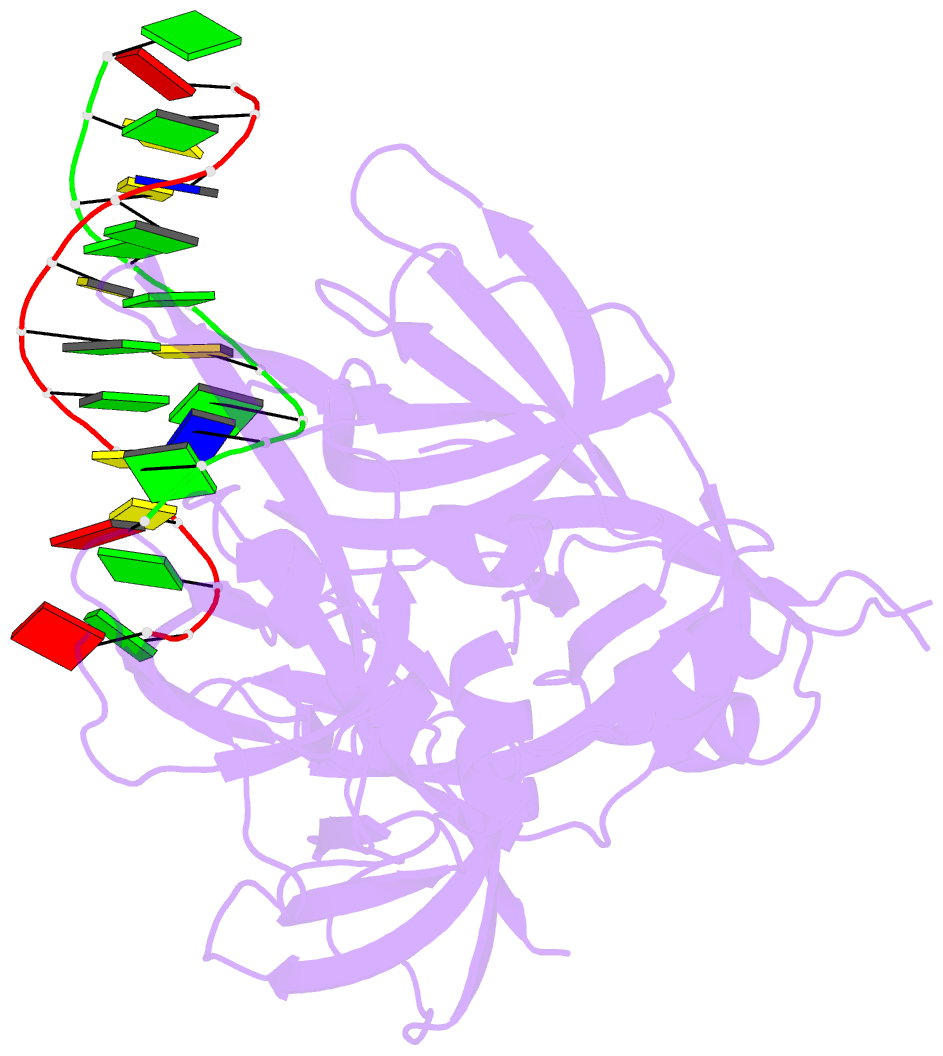
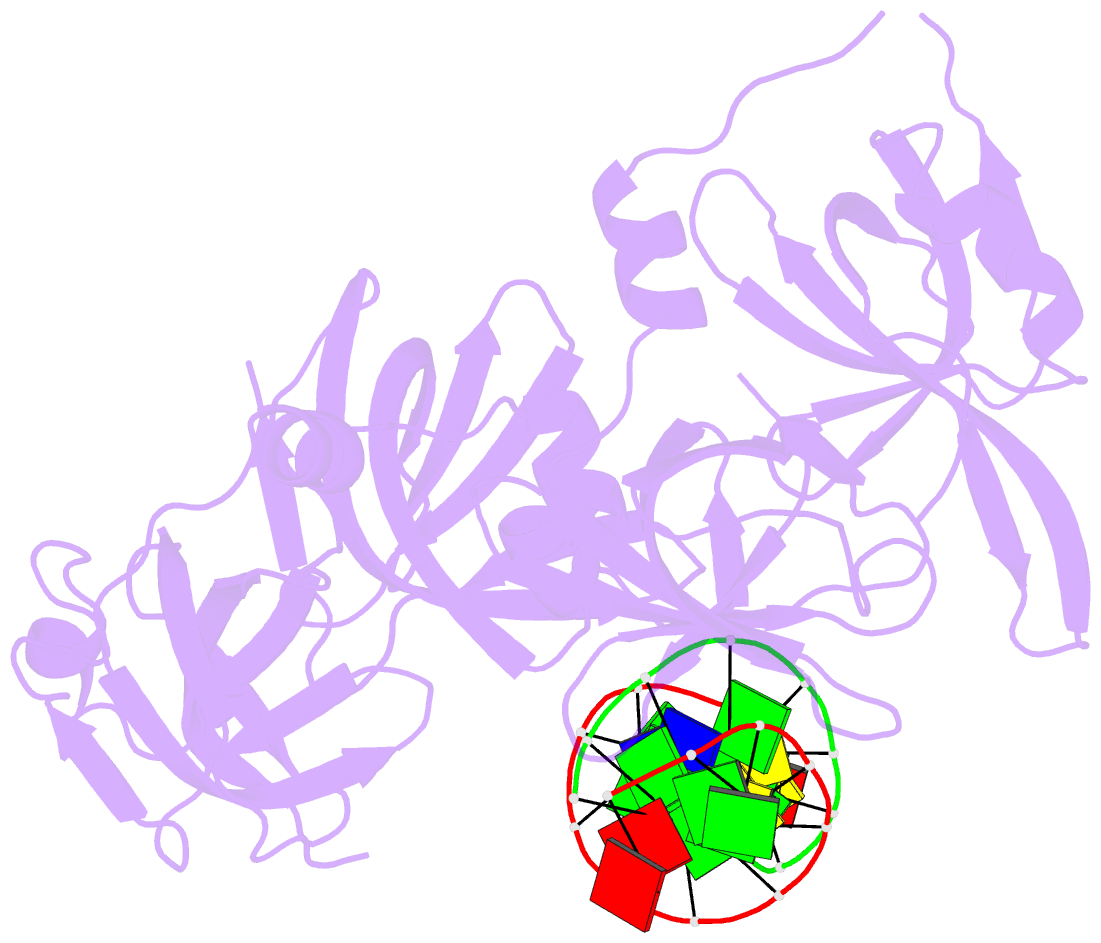
- Summary
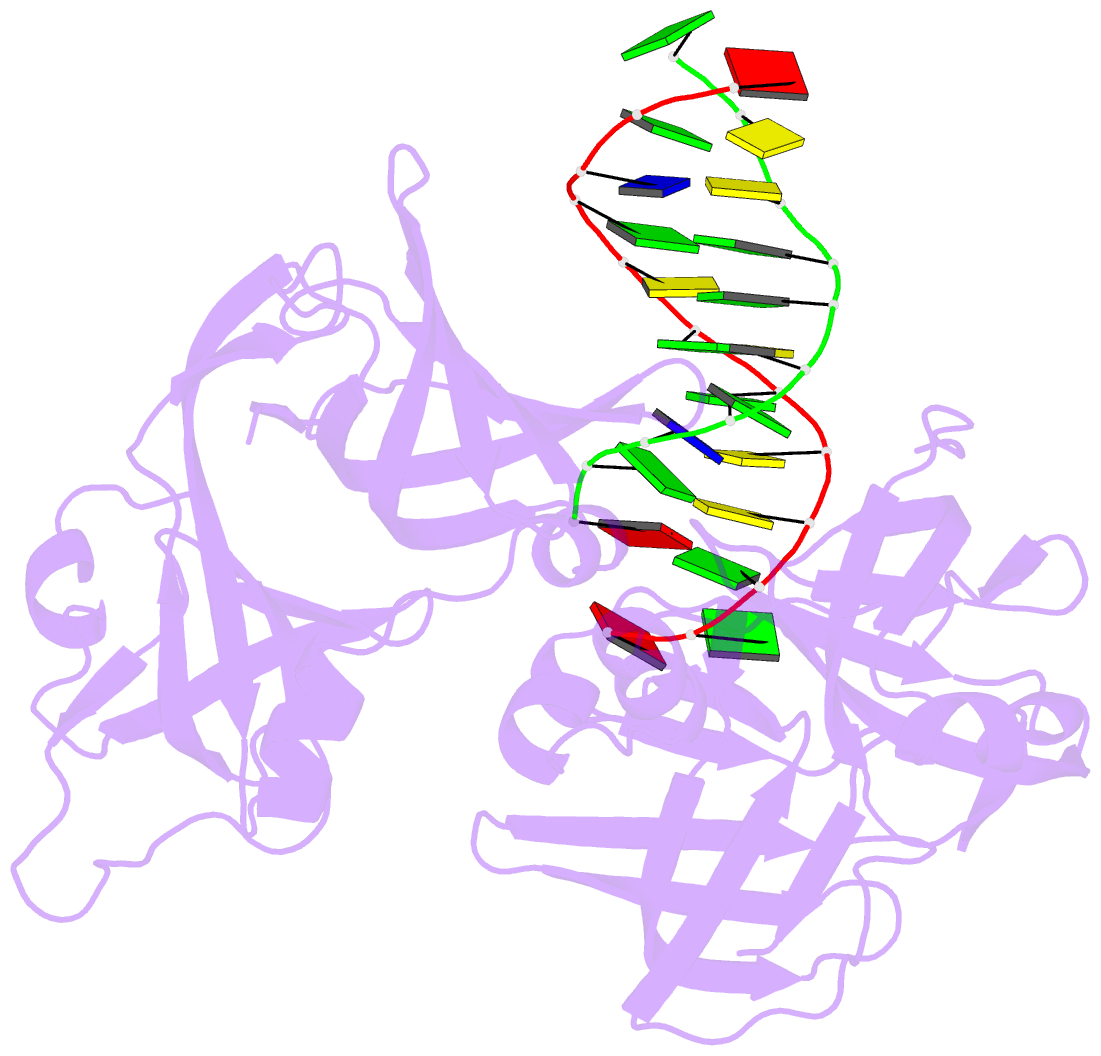
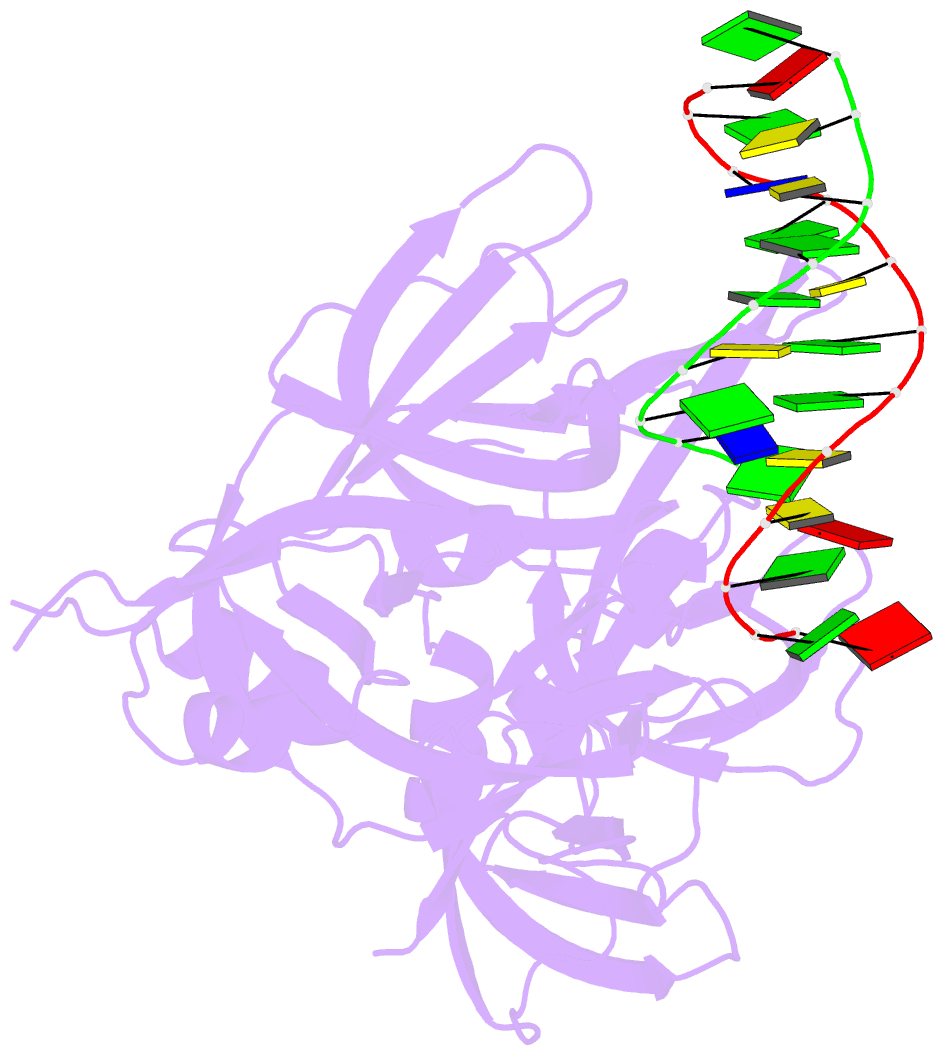
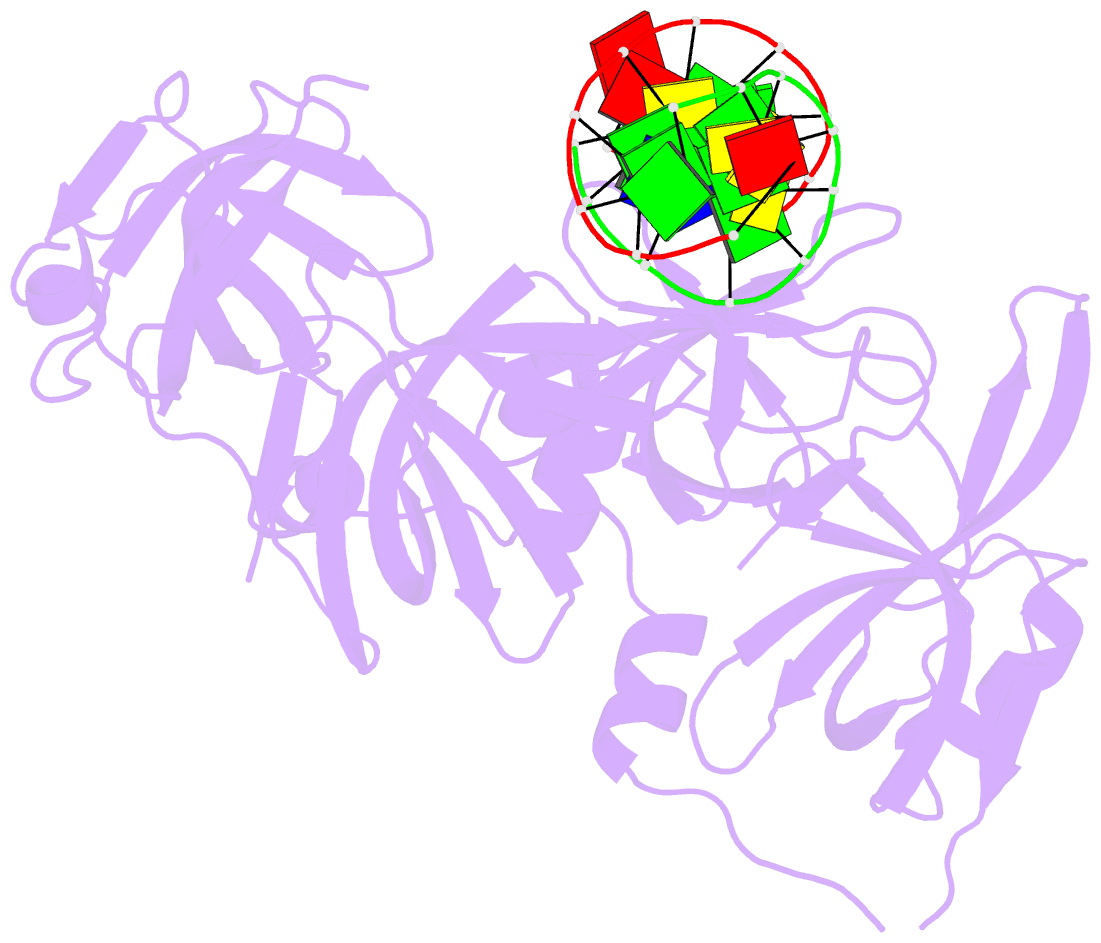
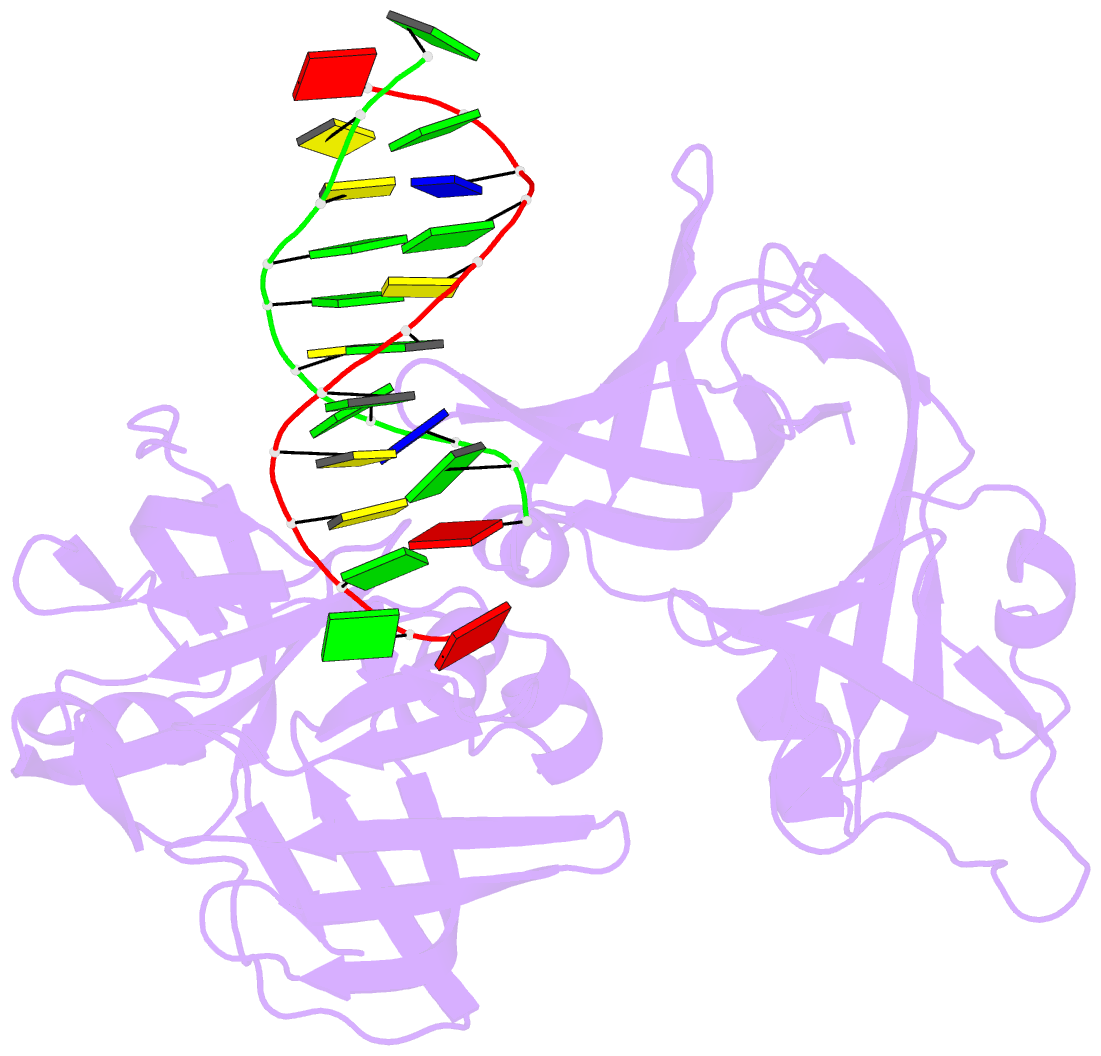
- Protein domain complex with ssDNA
- Reference
- Ni X, Ru H, Ma F, Zhao L, Shaw N, Feng Y, Ding W, Gong W, Wang Q, Ouyang S, Cheng G, Liu ZJ (2016): "New insights into the structural basis of DNA recognition by HINa and HINb domains of IFI16." J Mol Cell Biol, 8, 51-61. doi: 10.1093/jmcb/mjv053.
- Abstract
- Interferon gamma-inducible protein 16 (IFI16) senses DNA in the cytoplasm and the nucleus by using two tandem hematopoietic interferon-inducible nuclear (HIN) domains, HINa and HINb, through the cooperative assembly of IFI16 filaments on double-stranded DNA (dsDNA). The role of HINa in sensing DNA is not clearly understood. Here, we describe the crystal structure of the HINa domain in complex with DNA at 2.55 Å resolution and provide the first insight into the mode of DNA binding by the HINa domain. The structure reveals the presence of two oligosaccharide/nucleotide-binding (OB) folds with a unique DNA-binding surface. HINa uses loop L45 of the canonical OB2 fold to bind to the DNA backbone. The dsDNA is recognized as two single strands of DNA. Interestingly, deletion of HINb compromises the ability of IFI16 to induce IFN-β, while HINa mutants impaired in DNA binding enhance the production of IFN-β. These results shed light on the roles of IFI16 HIN domains in DNA recognition and innate immune responses.