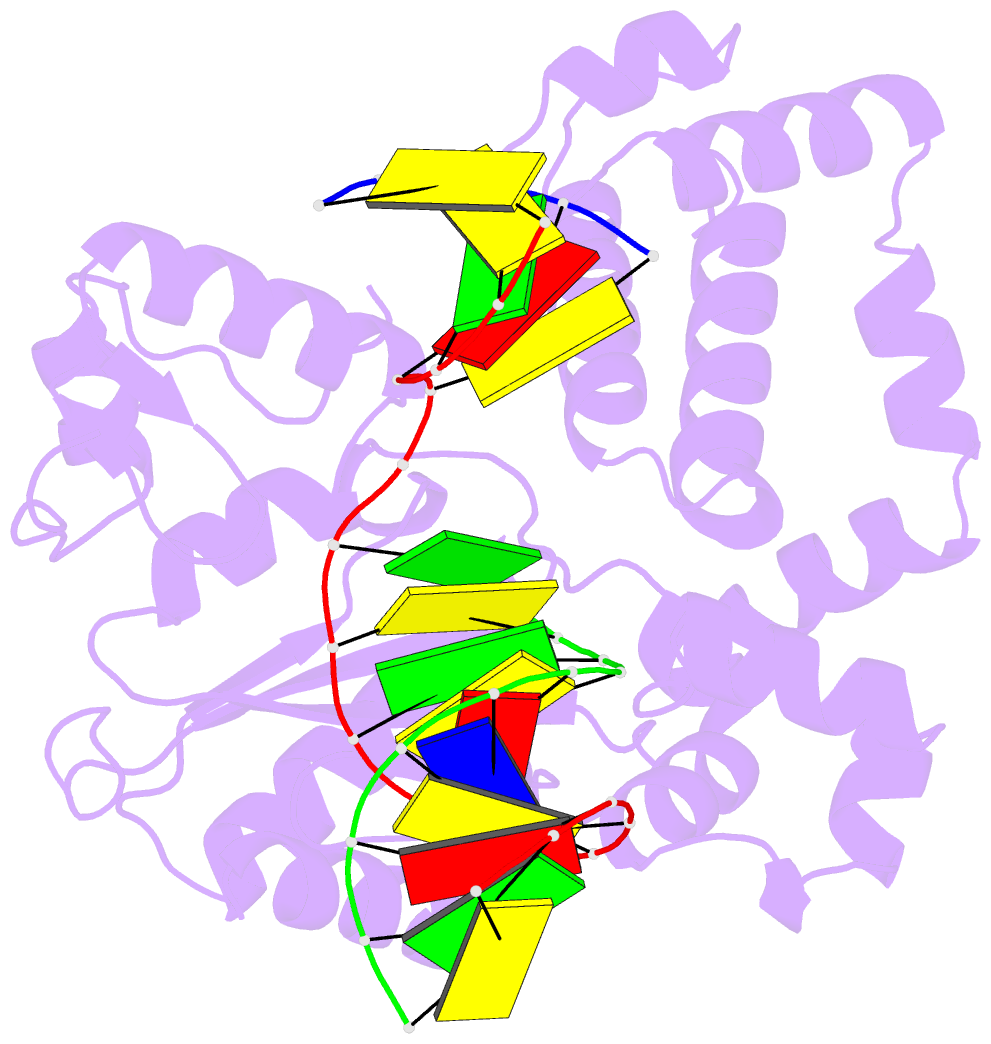
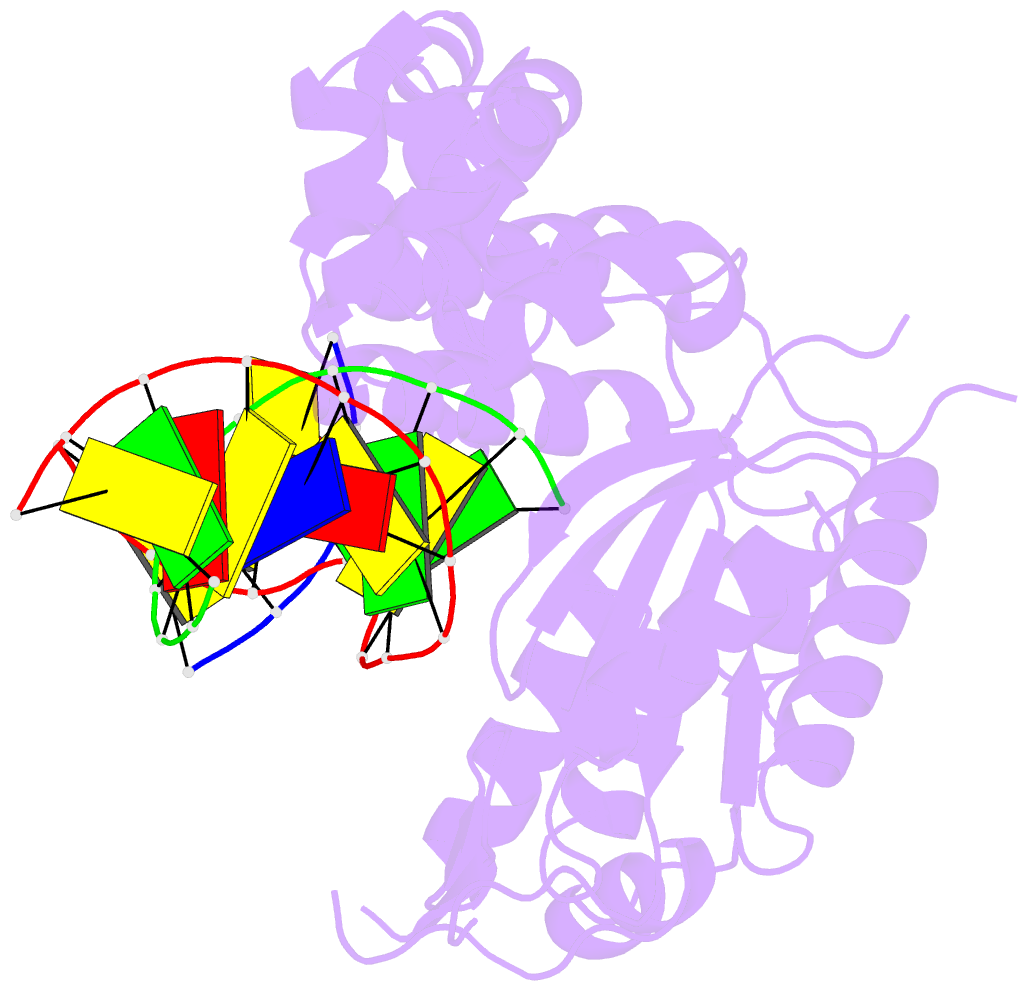
Summary information and primary citation
- PDB-id
- 4ppx; DSSR-derived features in text and JSON formats
- Class
- transferase, lyase-DNA
- Method
- X-ray (2.08 Å)
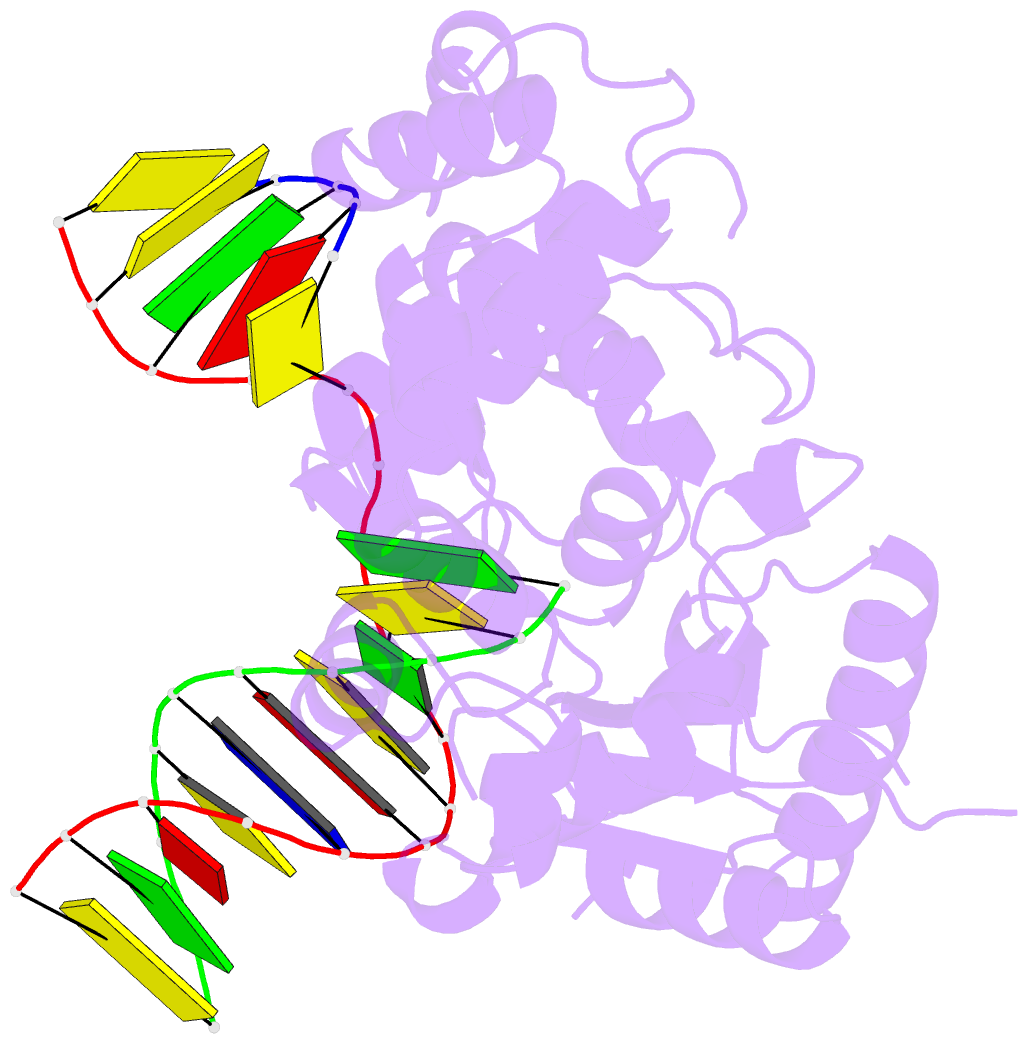
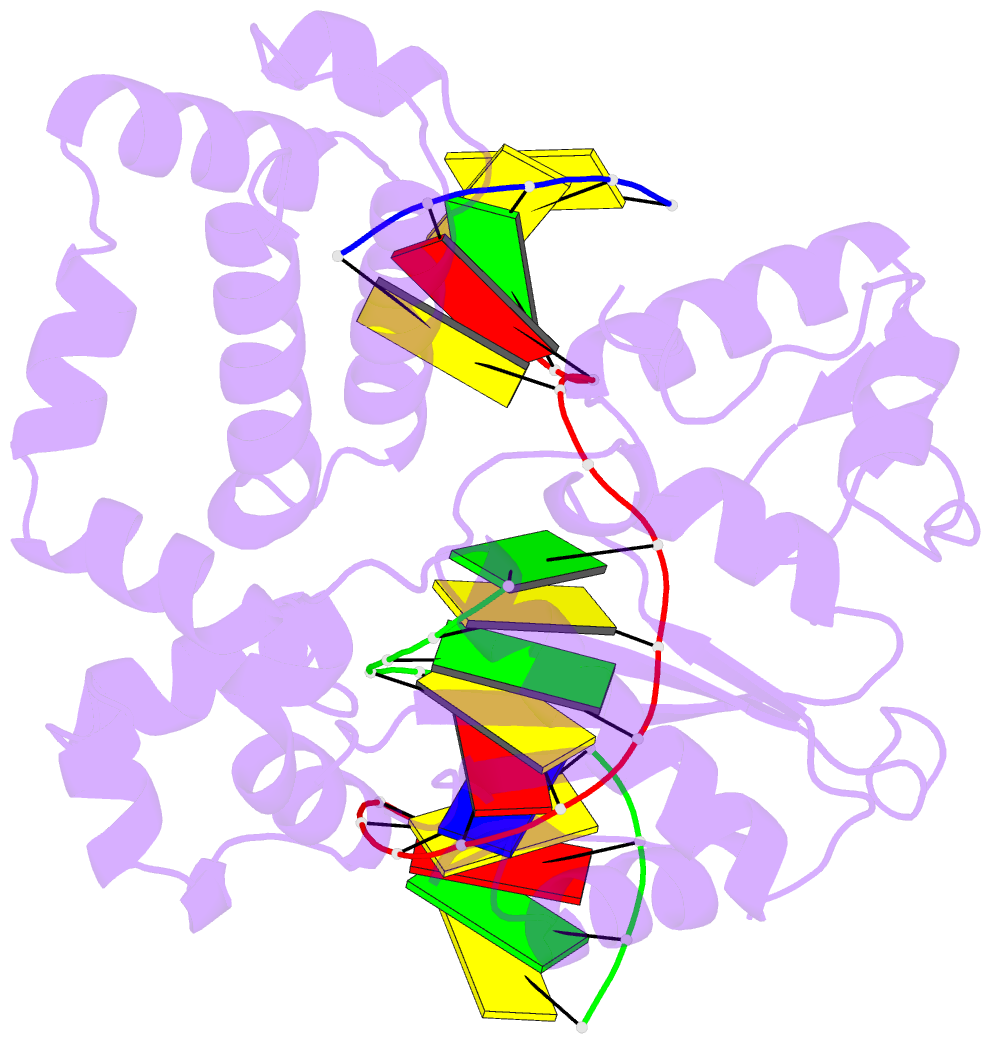
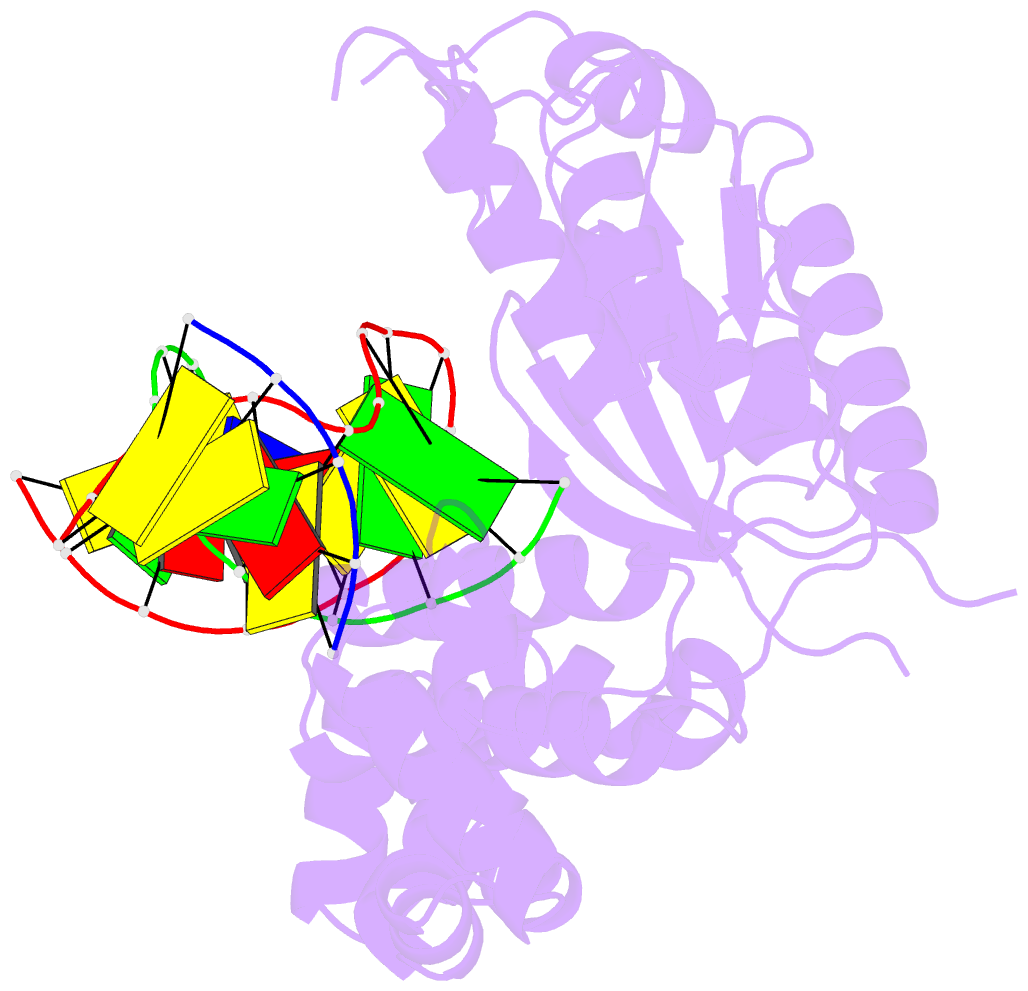
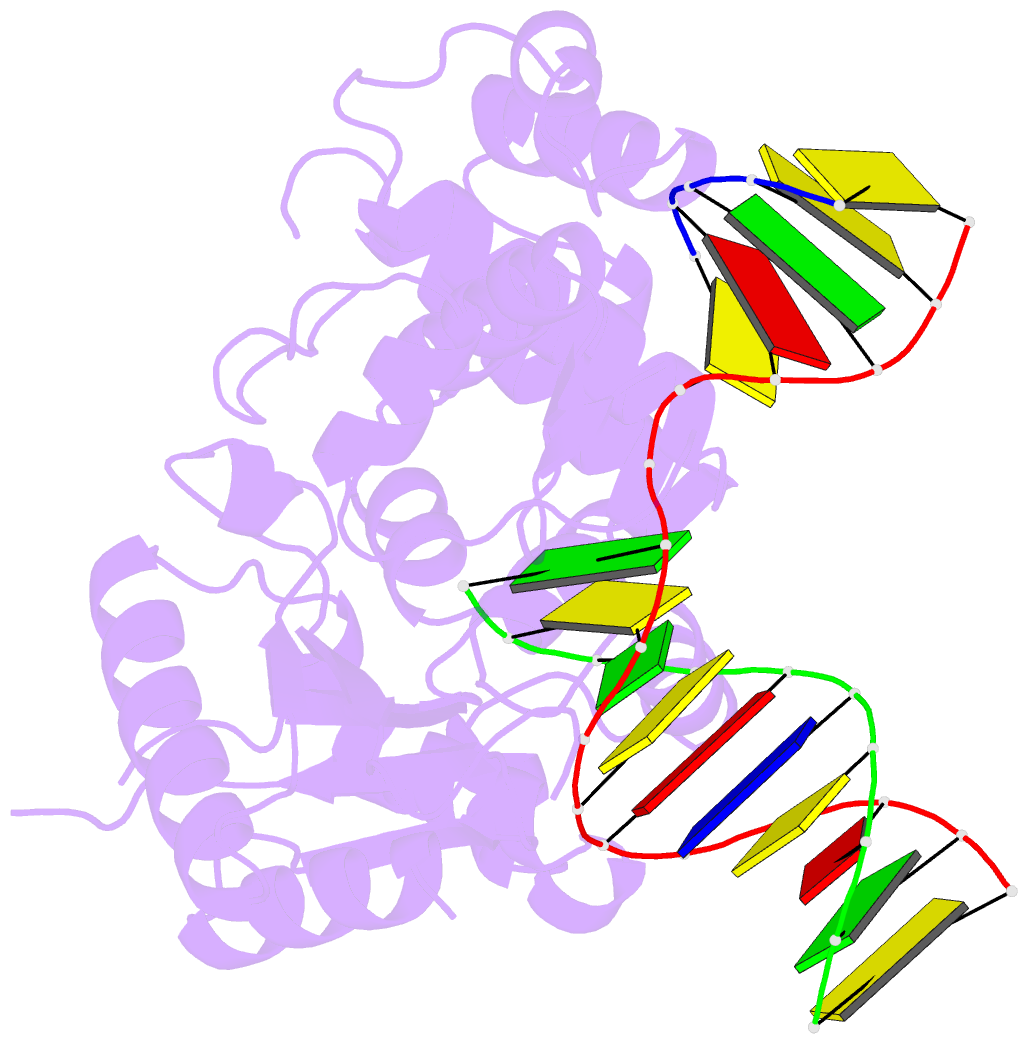
- Summary
- DNA polymerase beta e295k with spiroiminodihydantoin in templating position
- Reference
- Eckenroth BE, Fleming AM, Sweasy JB, Burrows CJ, Doublie S (2014): "Crystal Structure of DNA Polymerase beta with DNA Containing the Base Lesion Spiroiminodihydantoin in a Templating Position." Biochemistry, 53, 2075-2077. doi: 10.1021/bi500270e.
- Abstract
- The first high-resolution crystal structure of spiroiminodihydantoin (dSp1) was obtained in the context of the DNA polymerase β active site and reveals two areas of significance. First, the structure verifies the recently determined S configuration at the spirocyclic carbon. Second, the distortion of the DNA duplex is similar to that of the single-oxidation product 8-oxoguanine. For both oxidized lesions, adaptation of the syn conformation results in similar backbone distortions in the DNA duplex. The resulting conformation positions the dSp1 A-ring as the base-pairing face whereas the B-ring of dSp1 protrudes into the major groove.