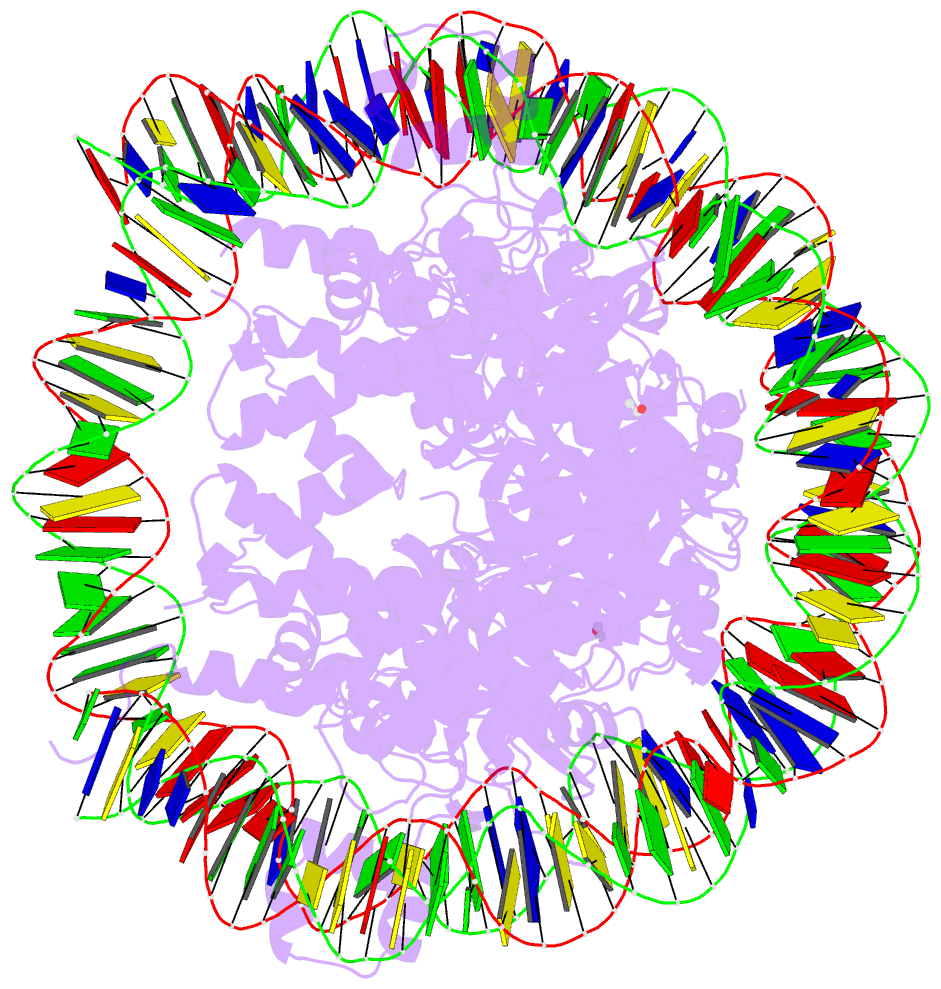
Summary information and primary citation
- PDB-id
- 4ld9; DSSR-derived features in text and JSON formats
- Class
- nuclear protein-transcription-DNA
- Method
- X-ray (3.306 Å)
- Summary
- Crystal structure of the n-terminally acetylated bah domain of sir3 bound to the nucleosome core particle
- Reference
- Arnaudo N, Fernandez IS, McLaughlin SH, Peak-Chew SY, Rhodes D, Martino F (2013): "The N-terminal acetylation of Sir3 stabilizes its binding to the nucleosome core particle." Nat.Struct.Mol.Biol., 20, 1119-1121. doi: 10.1038/nsmb.2641.
- Abstract
- The N-terminal acetylation of Sir3 is essential for heterochromatin establishment and maintenance in yeast, but its mechanism of action is unknown. The crystal structure of the N-terminally acetylated BAH domain of Saccharomyces cerevisiae Sir3 bound to the nucleosome core particle reveals that the N-terminal acetylation stabilizes the interaction of Sir3 with the nucleosome. Additionally, we present a new method for the production of protein-nucleosome complexes for structural analysis.