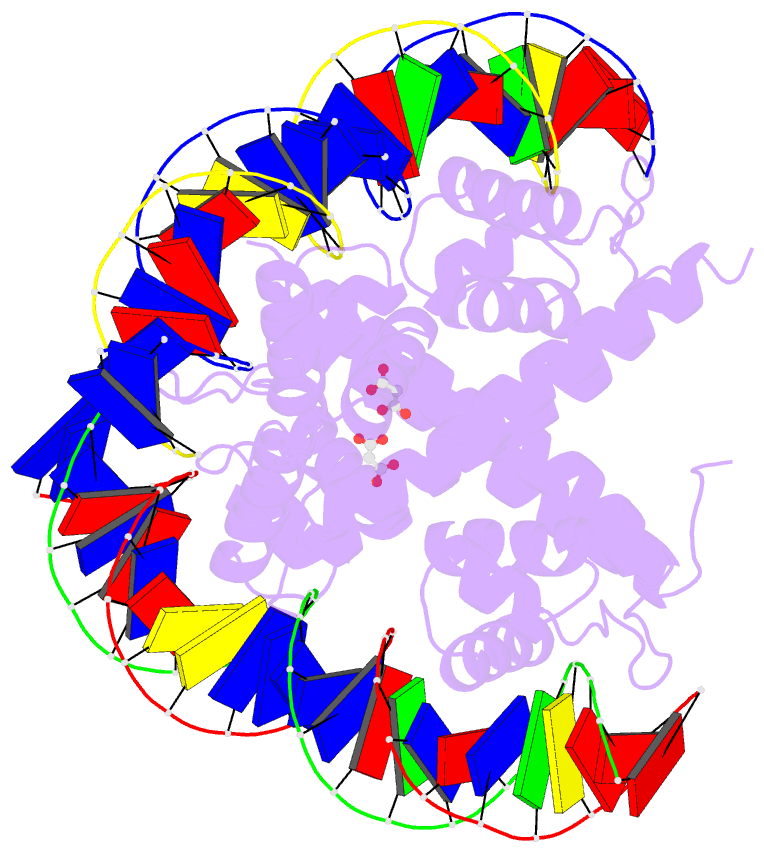
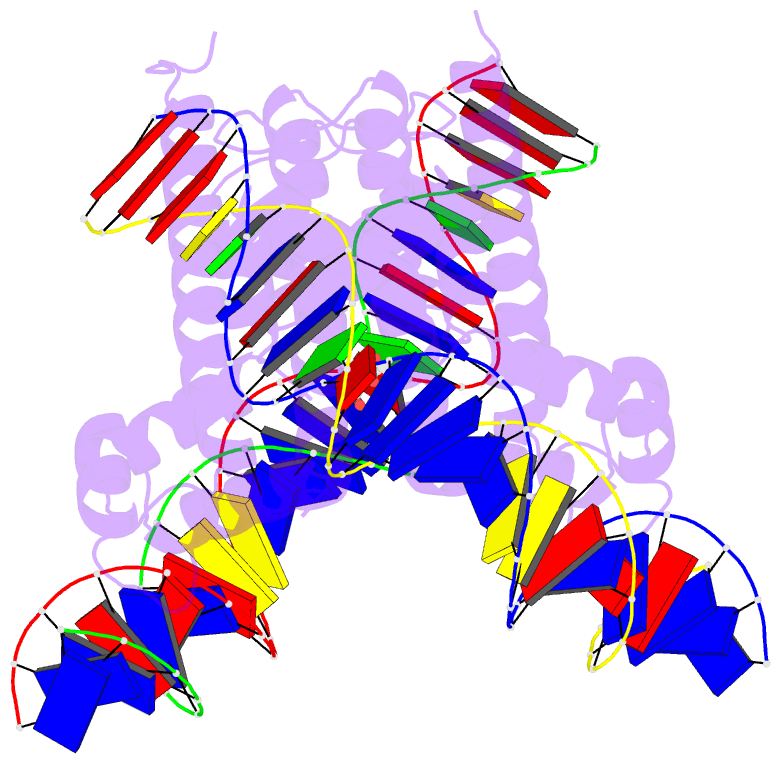
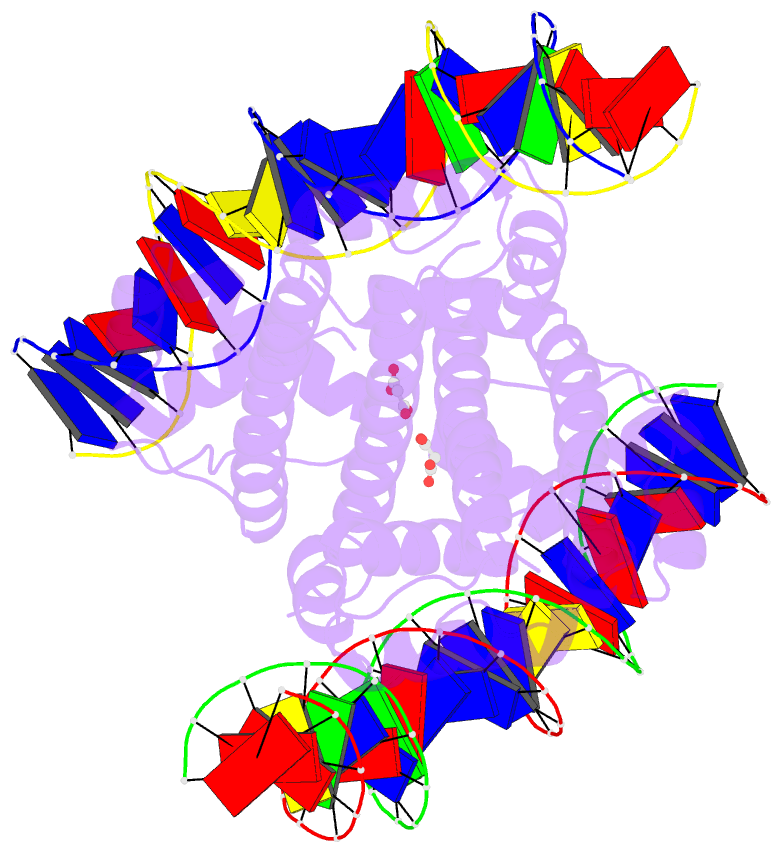
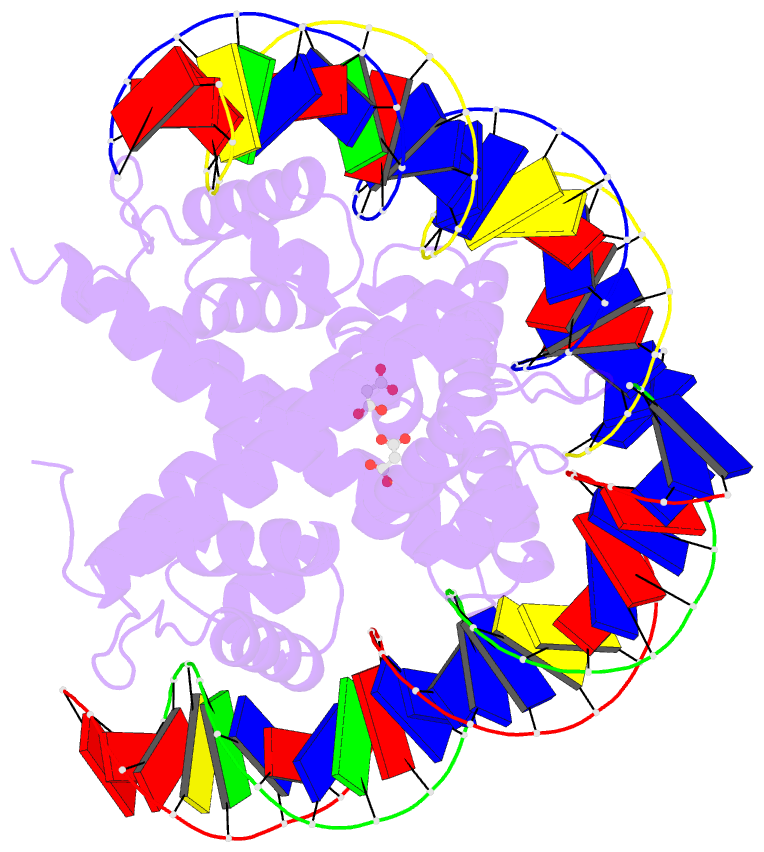
Summary information and primary citation
- PDB-id
-
4ihs;
SNAP-derived features in text and
JSON formats
- Class
- transcription-DNA
- Method
- X-ray (3.1 Å)
- Summary
- Crystal structure of benm_dbd-catb site 1 DNA
complex
- Reference
-
Alanazi AM, Neidle EL, Momany C (2013): "The
DNA-binding domain of BenM reveals the structural basis
for the recognition of a T-N11-A sequence motif by
LysR-type transcriptional regulators." Acta
Crystallogr.,Sect.D, 69, 1995-2007.
doi: 10.1107/S0907444913017320.
- Abstract
- LysR-type transcriptional regulators (LTTRs) play
critical roles in metabolism and constitute the largest
family of bacterial regulators. To understand protein-DNA
interactions, atomic structures of the DNA-binding domain
and linker-helix regions of a prototypical LTTR, BenM, were
determined by X-ray crystallography. BenM structures with
and without bound DNA reveal a set of highly conserved
amino acids that interact directly with DNA bases. At the
N-terminal end of the recognition helix (α3) of a
winged-helix-turn-helix DNA-binding motif, several residues
create hydrophobic pockets (Pro30, Pro31 and Ser33). These
pockets interact with the methyl groups of two thymines in
the DNA-recognition motif and its complementary strand,
T-N11-A. This motif usually includes some dyad symmetry, as
exemplified by a sequence that binds two subunits of a BenM
tetramer (ATAC-N7-GTAT). Gln29 forms hydrogen bonds to
adenine in the first position of the recognition half-site
(ATAC). Another hydrophobic pocket defined by Ala28, Pro30
and Pro31 interacts with the methyl group of thymine,
complementary to the base at the third position of the
half-site. Arg34 interacts with the complementary base of
the 3' position. Arg53, in the wing, provides AT-tract
recognition in the minor groove. For DNA recognition, LTTRs
use highly conserved interactions between amino acids and
nucleotide bases as well as numerous less-conserved
secondary interactions.