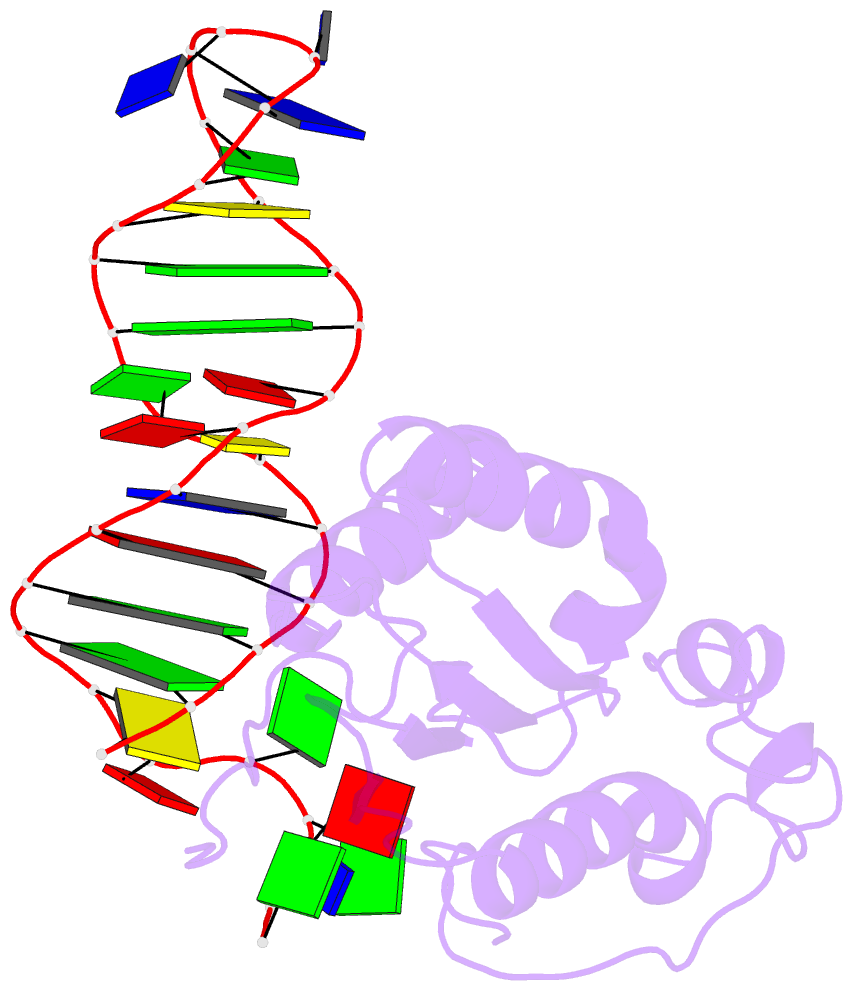
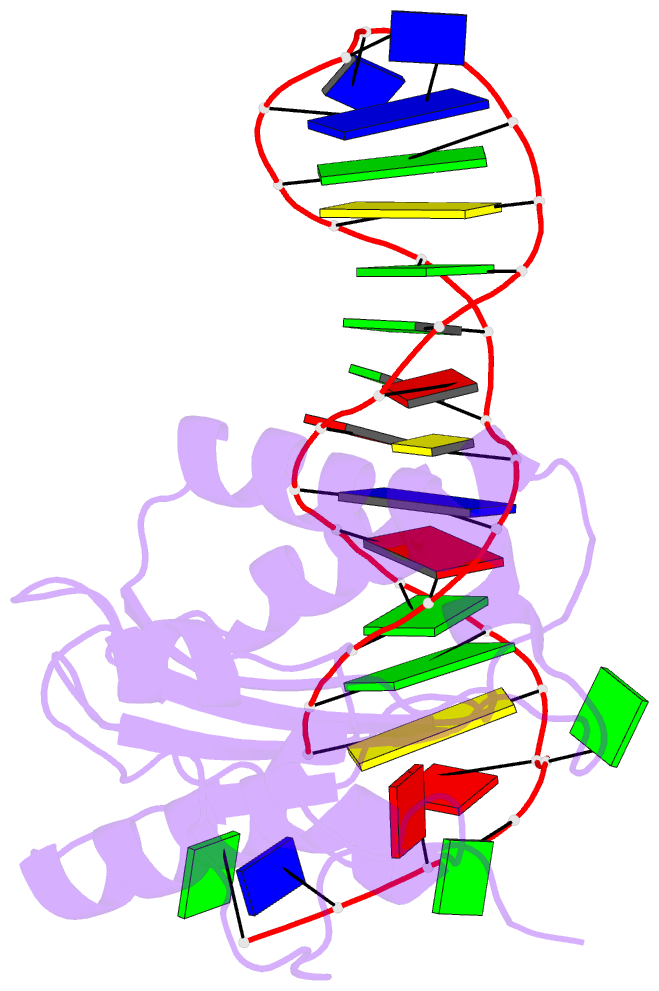
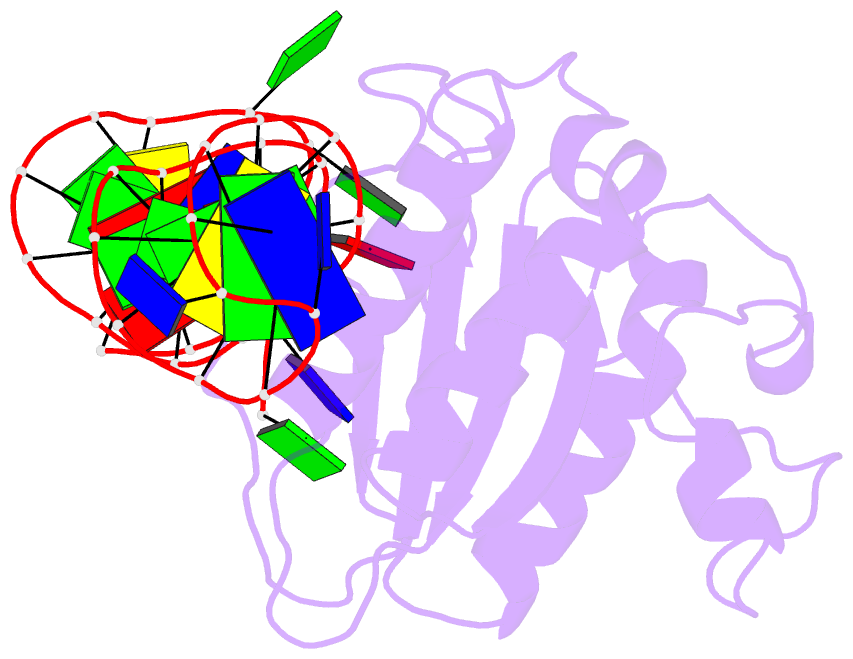
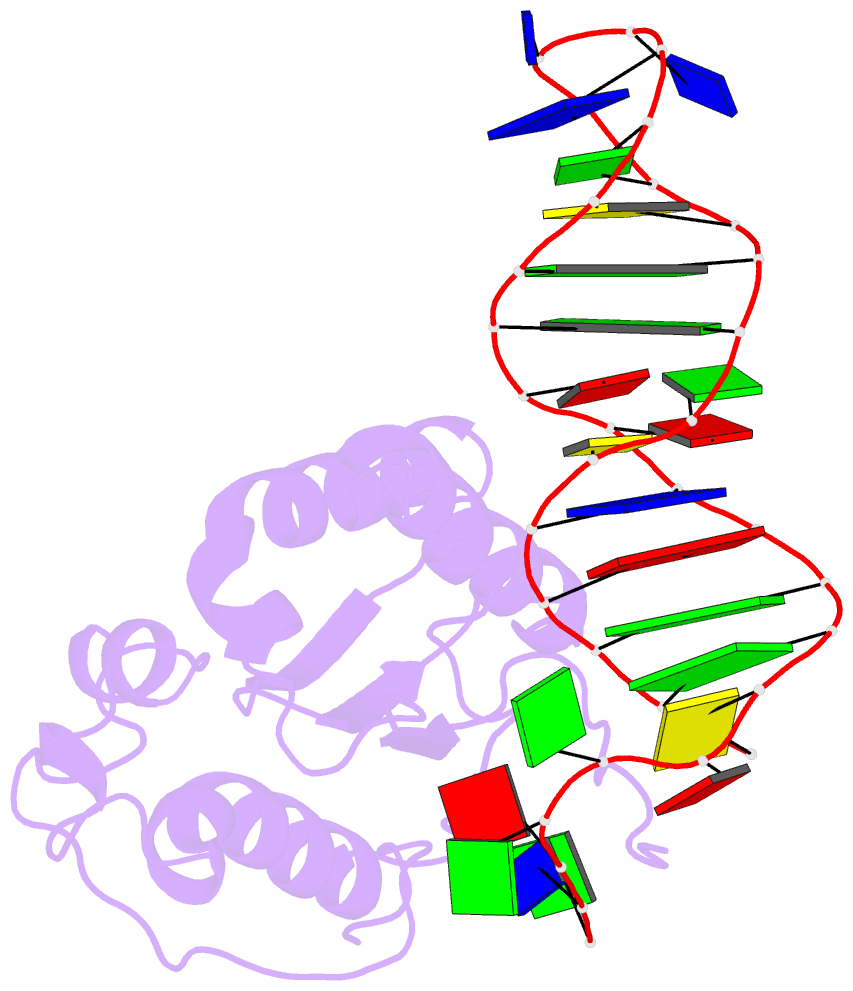
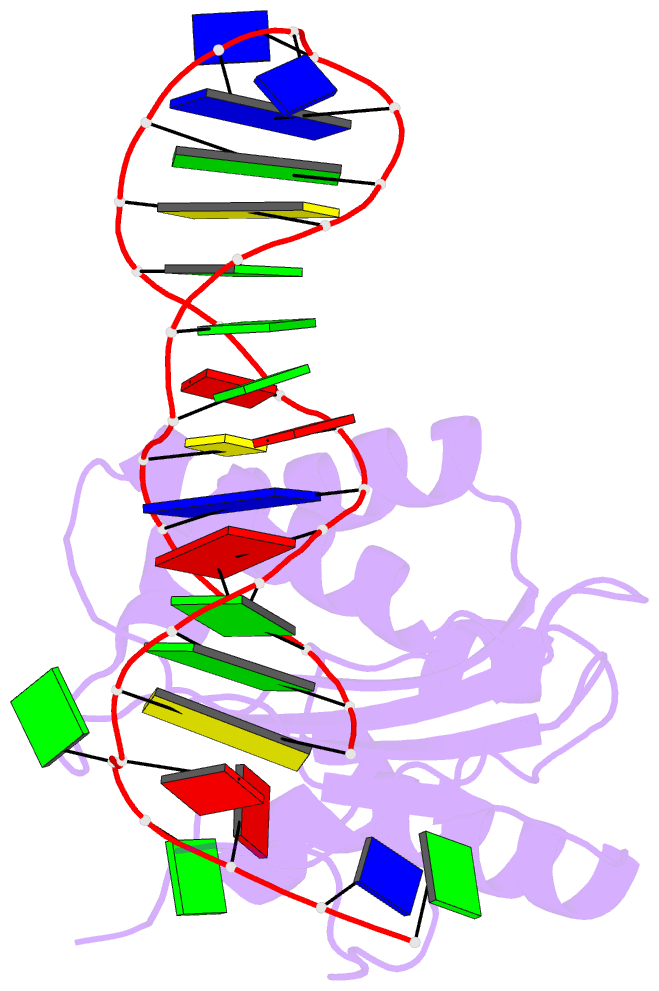
Summary information and primary citation
- PDB-id
- 4er8; DSSR-derived features in text and JSON formats
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.6 Å)
- Summary
- Structure of the rep associates tyrosine transposase bound to a rep hairpin
- Reference
- Messing SA, Ton-Hoang B, Hickman AB, McCubbin AJ, Peaslee GF, Ghirlando R, Chandler M, Dyda F (2012): "The processing of repetitive extragenic palindromes: the structure of a repetitive extragenic palindrome bound to its associated nuclease." Nucleic Acids Res., 40, 9964-9979. doi: 10.1093/nar/gks741.
- Abstract
- Extragenic sequences in genomes, such as microRNA and CRISPR, are vital players in the cell. Repetitive extragenic palindromic sequences (REPs) are a class of extragenic sequences, which form nucleotide stem-loop structures. REPs are found in many bacterial species at a high copy number and are important in regulation of certain bacterial functions, such as Integration Host Factor recruitment and mRNA turnover. Although a new clade of putative transposases (RAYTs or TnpA(REP)) is often associated with an increase in these repeats, it is not clear how these proteins might have directed amplification of REPs. We report here the structure to 2.6 Å of TnpA(REP) from Escherichia coli MG1655 bound to a REP. Sequence analysis showed that TnpA(REP) is highly related to the IS200/IS605 family, but in contrast to IS200/IS605 transposases, TnpA(REP) is a monomer, is auto-inhibited and is active only in manganese. These features suggest that, relative to IS200/IS605 transposases, it has evolved a different mechanism for the movement of discrete segments of DNA and has been severely down-regulated, perhaps to prevent REPs from sweeping through genomes.