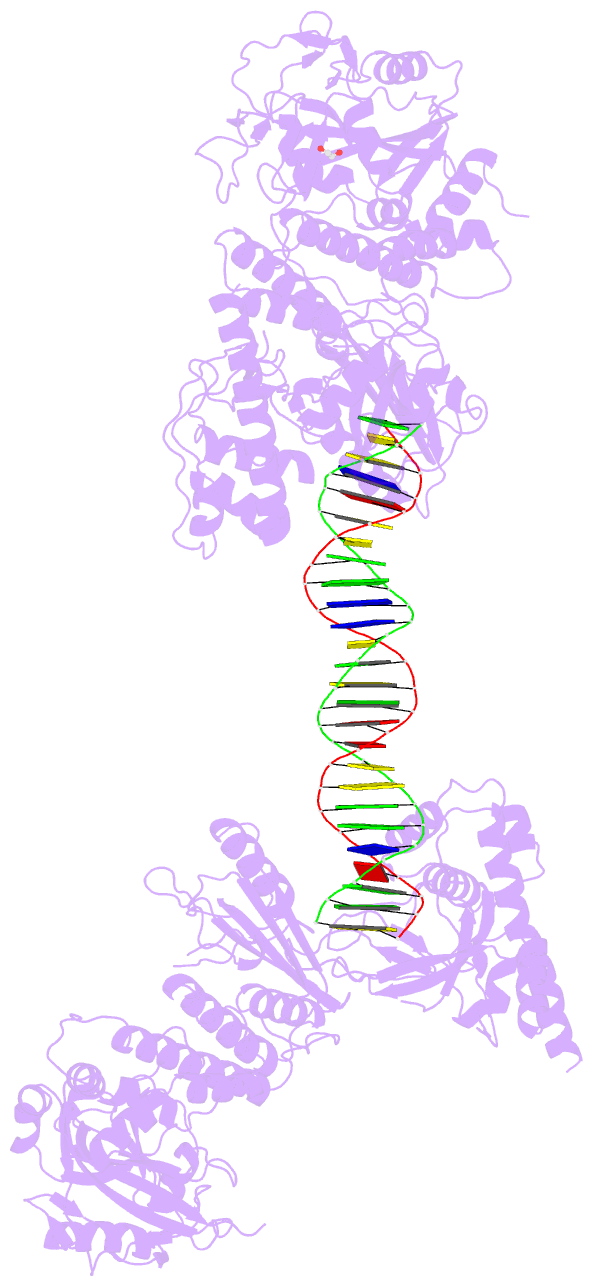
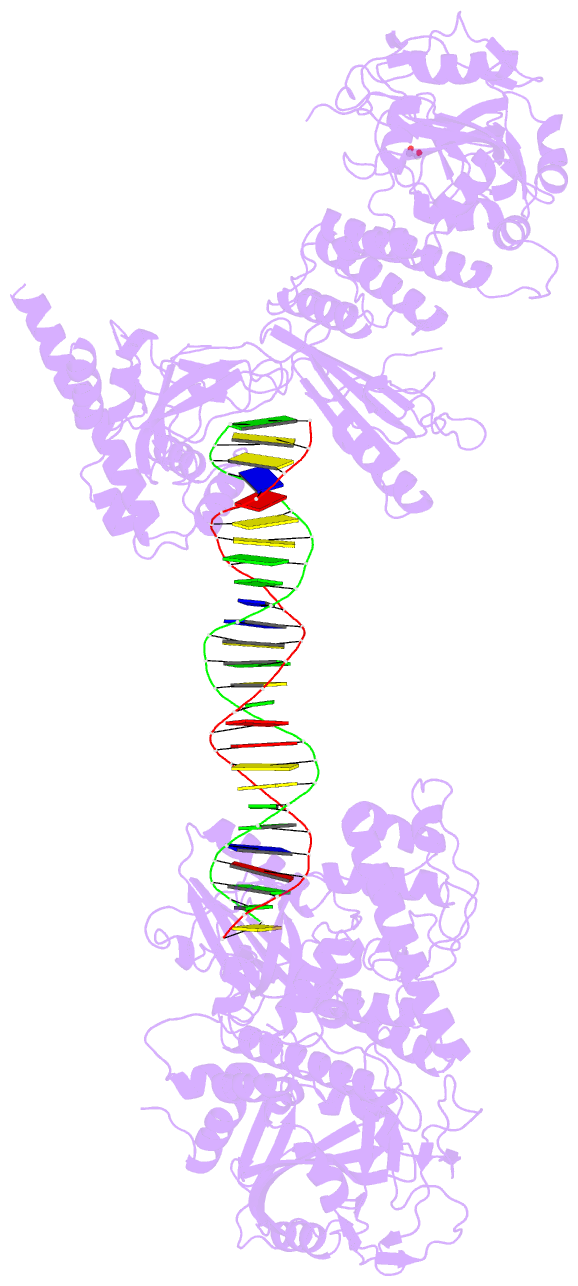
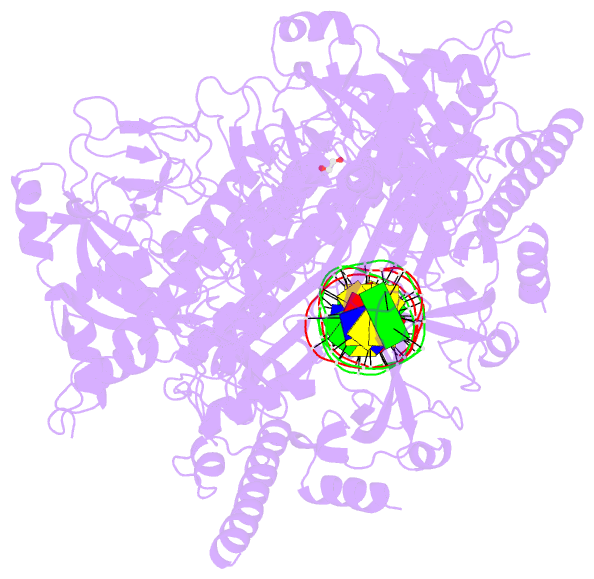
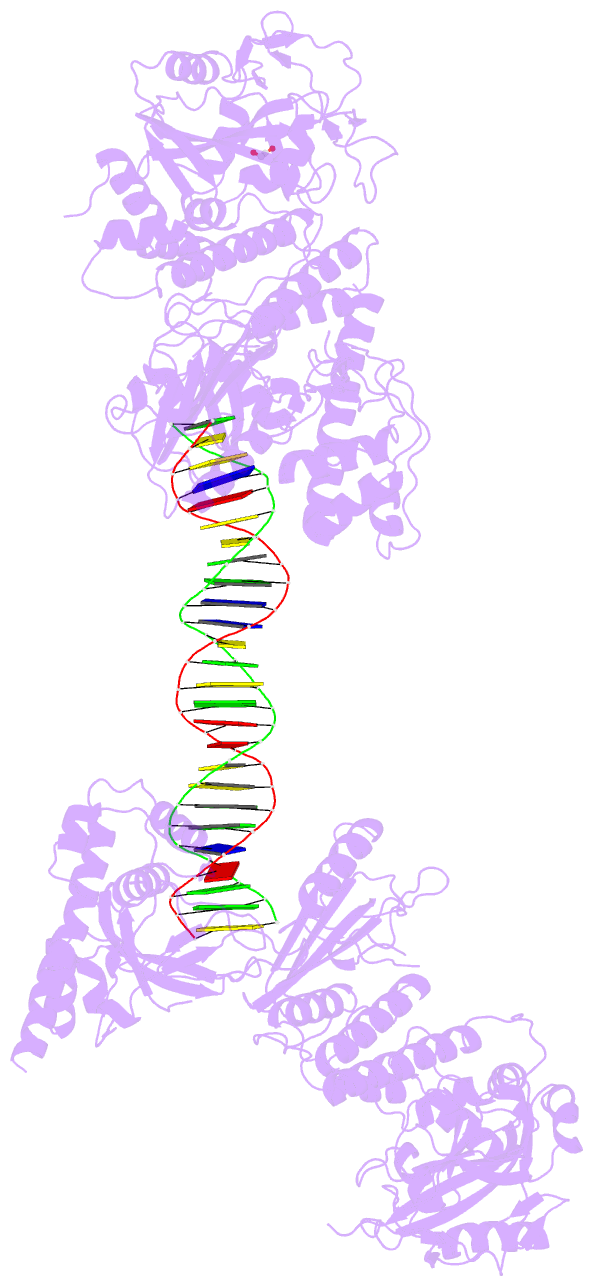
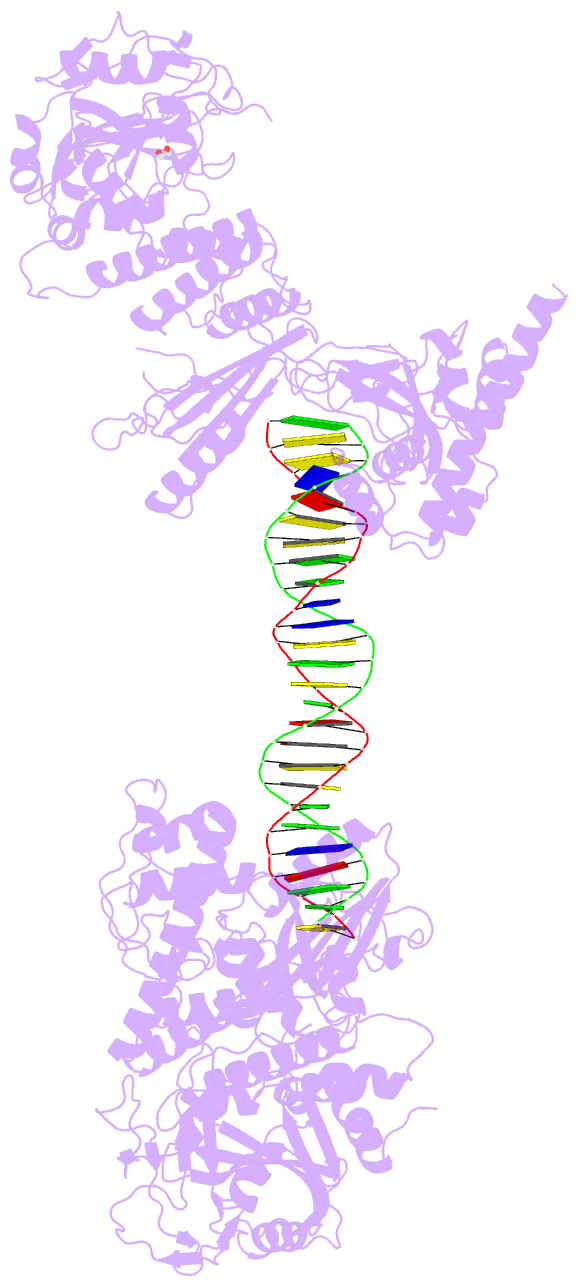
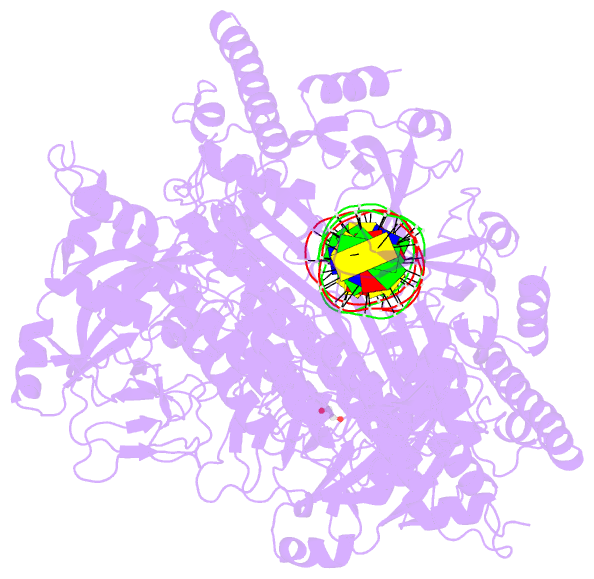
Summary information and primary citation
- PDB-id
- 4dqy; DSSR-derived features in text and JSON formats
- Class
- transferase-DNA
- Method
- X-ray (3.25 Å)
- Summary
- Structure of human parp-1 bound to a DNA double strand break
- Reference
- Langelier MF, Planck JL, Roy S, Pascal JM (2012): "Structural basis for DNA damage-dependent poly(ADP-ribosyl)ation by human PARP-1." Science, 336, 728-732. doi: 10.1126/science.1216338.
- Abstract
- Poly(ADP-ribose) polymerase-1 (PARP-1) (ADP, adenosine diphosphate) has a modular domain architecture that couples DNA damage detection to poly(ADP-ribosyl)ation activity through a poorly understood mechanism. Here, we report the crystal structure of a DNA double-strand break in complex with human PARP-1 domains essential for activation (Zn1, Zn3, WGR-CAT). PARP-1 engages DNA as a monomer, and the interaction with DNA damage organizes PARP-1 domains into a collapsed conformation that can explain the strong preference for automodification. The Zn1, Zn3, and WGR domains collectively bind to DNA, forming a network of interdomain contacts that links the DNA damage interface to the catalytic domain (CAT). The DNA damage-induced conformation of PARP-1 results in structural distortions that destabilize the CAT. Our results suggest that an increase in CAT protein dynamics underlies the DNA-dependent activation mechanism of PARP-1.