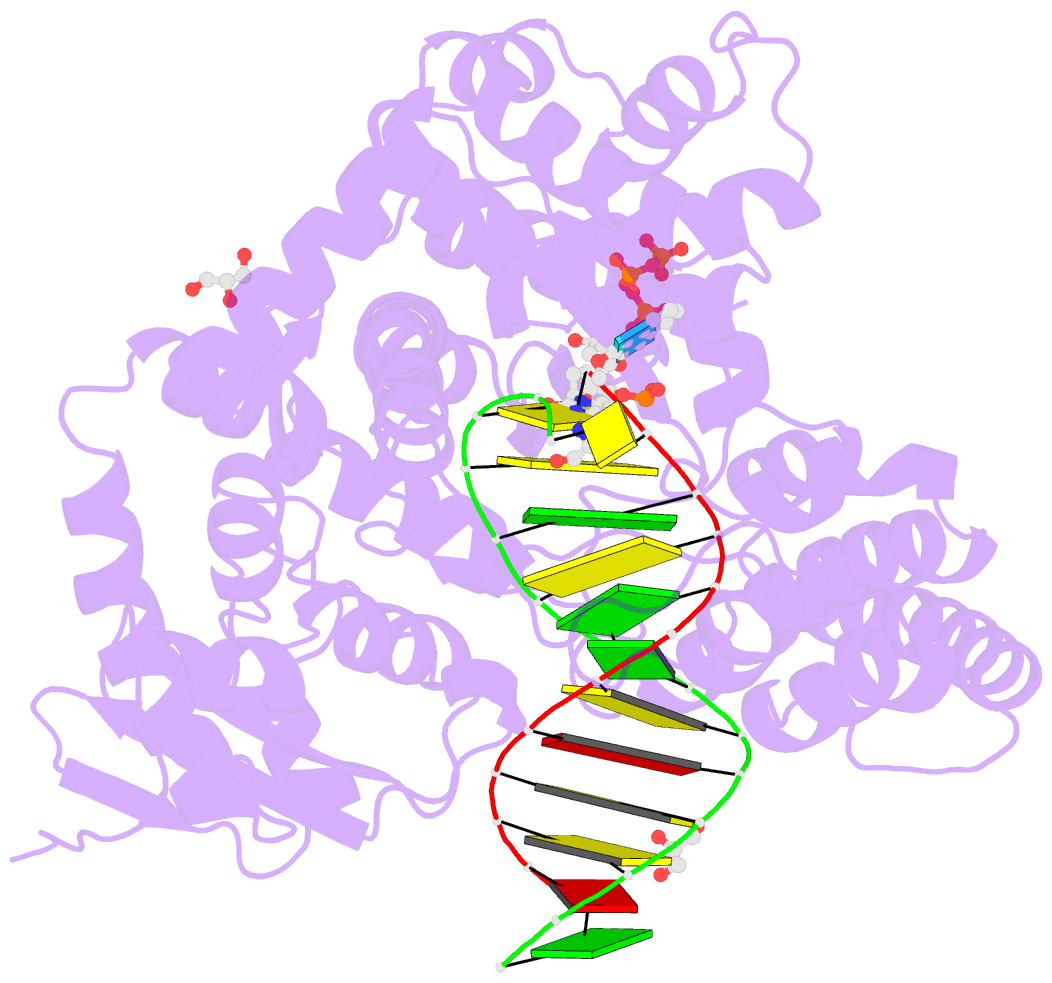
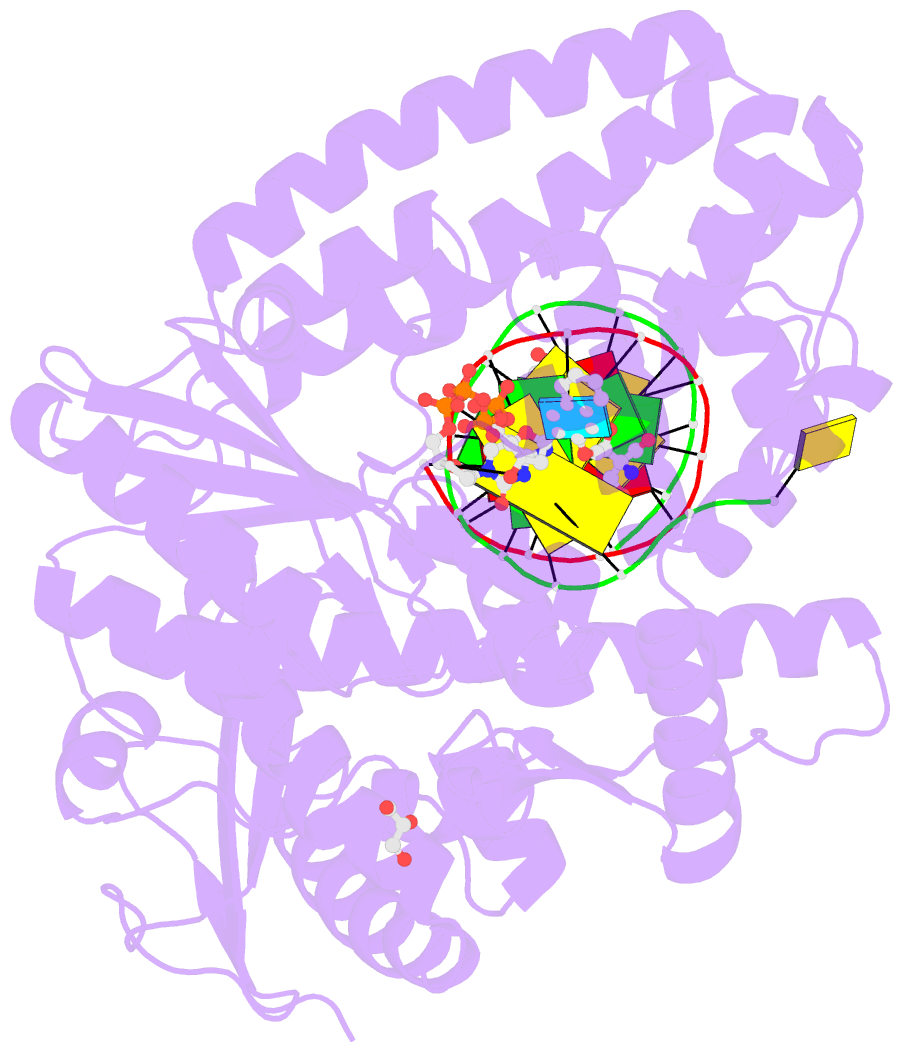
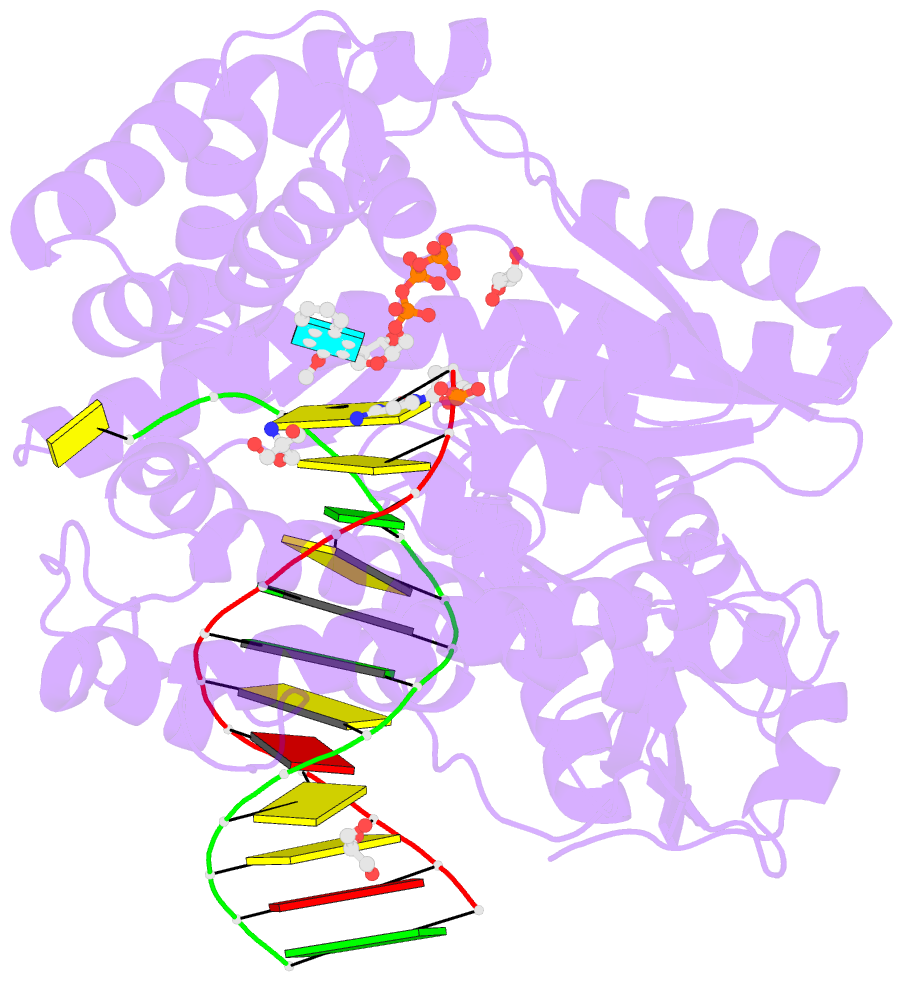
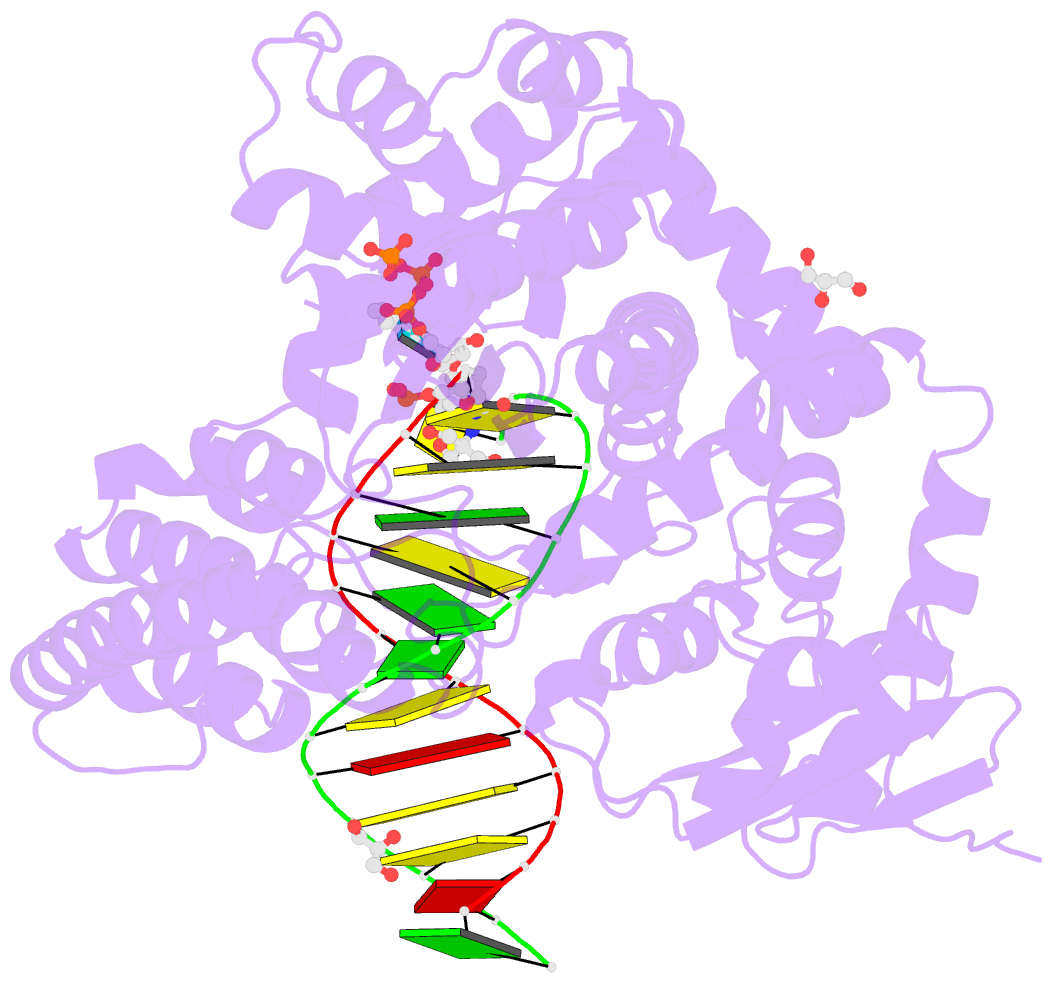
Summary information and primary citation
- PDB-id
- 4c8k; DSSR-derived features in text and JSON formats
- Class
- transferase-DNA
- Method
- X-ray (2.17 Å)
- Summary
- Crystal structure of the large fragment of DNA polymerase i from thermus aquaticus in a partially closed complex with the artificial base pair d5sics-dnamtp
- Reference
- Betz K, Malyshev DA, Lavergne T, Welte W, Diederichs K, Romesberg FE, Marx A (2013): "Structural Insights Into DNA Replication without Hydrogen Bonds." J.Am.Chem.Soc., 135, 18637. doi: 10.1021/JA409609J.
- Abstract
- The genetic alphabet is composed of two base pairs, and the development of a third, unnatural base pair would increase the genetic and chemical potential of DNA. d5SICS-dNaM is one of the most efficiently replicated unnatural base pairs identified to date, but its pairing is mediated by only hydrophobic and packing forces, and in free duplex DNA it forms a cross-strand intercalated structure that makes its efficient replication difficult to understand. Recent studies of the KlenTaq DNA polymerase revealed that the insertion of d5SICSTP opposite dNaM proceeds via a mutually induced-fit mechanism, where the presence of the triphosphate induces the polymerase to form the catalytically competent closed structure, which in turn induces the pairing nucleotides of the developing unnatural base pair to adopt a planar Watson-Crick-like structure. To understand the remaining steps of replication, we now report the characterization of the prechemistry complexes corresponding to the insertion of dNaMTP opposite d5SICS, as well as multiple postchemistry complexes in which the already formed unnatural base pair is positioned at the postinsertion site. Unlike with the insertion of d5SICSTP opposite dNaM, addition of dNaMTP does not fully induce the formation of the catalytically competent closed state. The data also reveal that once synthesized and translocated to the postinsertion position, the unnatural nucleobases again intercalate. Two modes of intercalation are observed, depending on the nature of the flanking nucleotides, and are each stabilized by different interactions with the polymerase, and each appear to reduce the affinity with which the next correct triphosphate binds. Thus, continued primer extension is limited by deintercalation and rearrangements with the polymerase active site that are required to populate the catalytically active, triphosphate bound conformation.