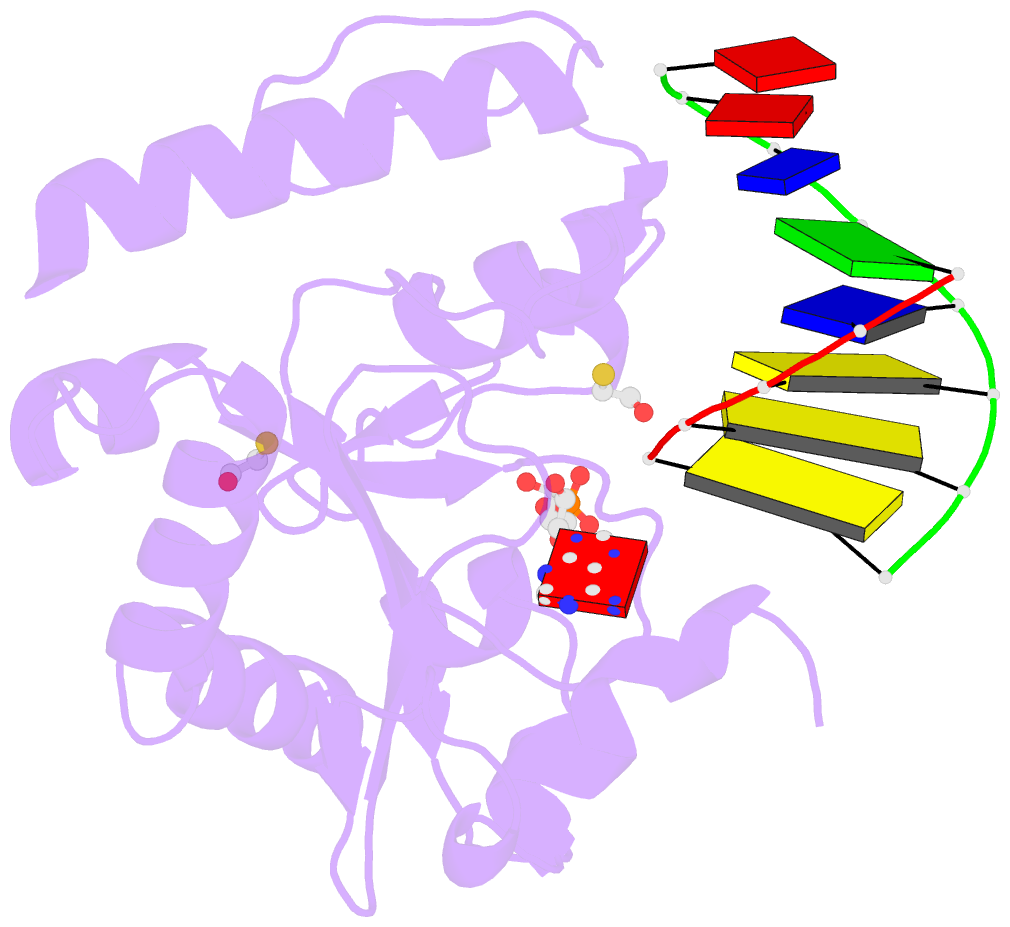
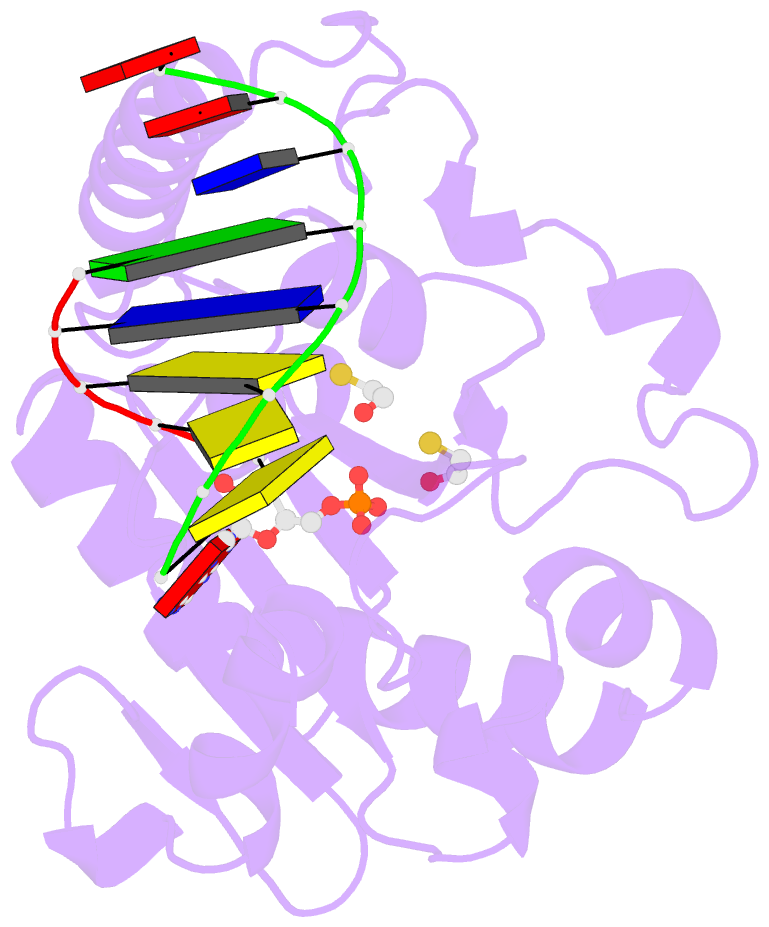
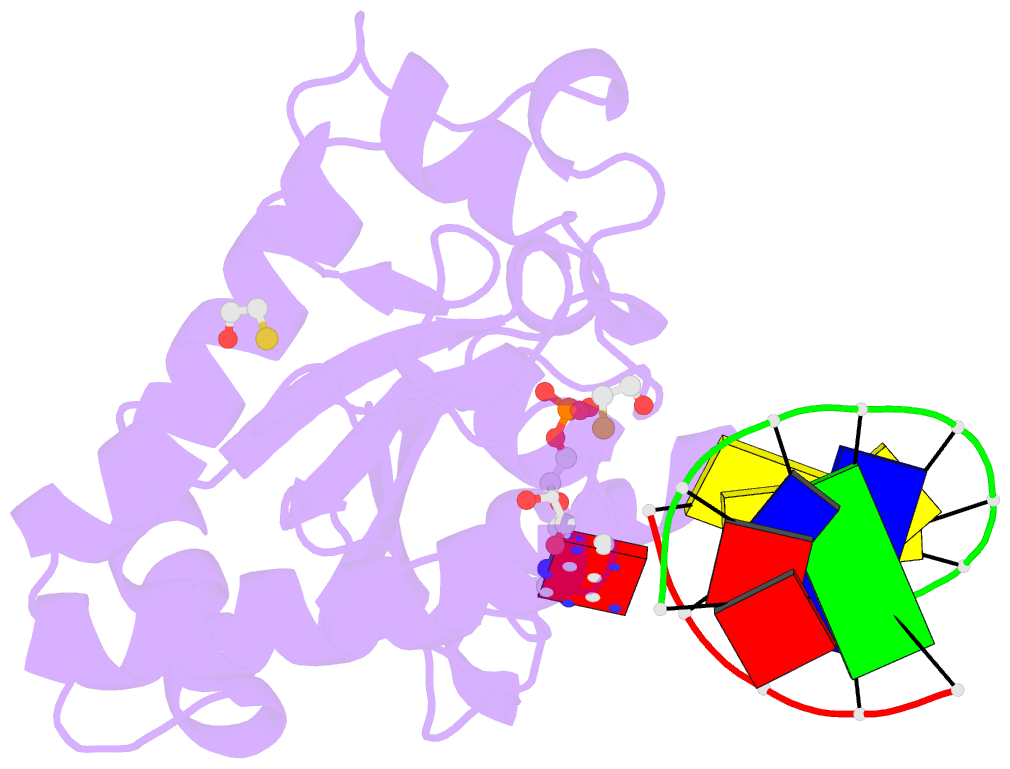
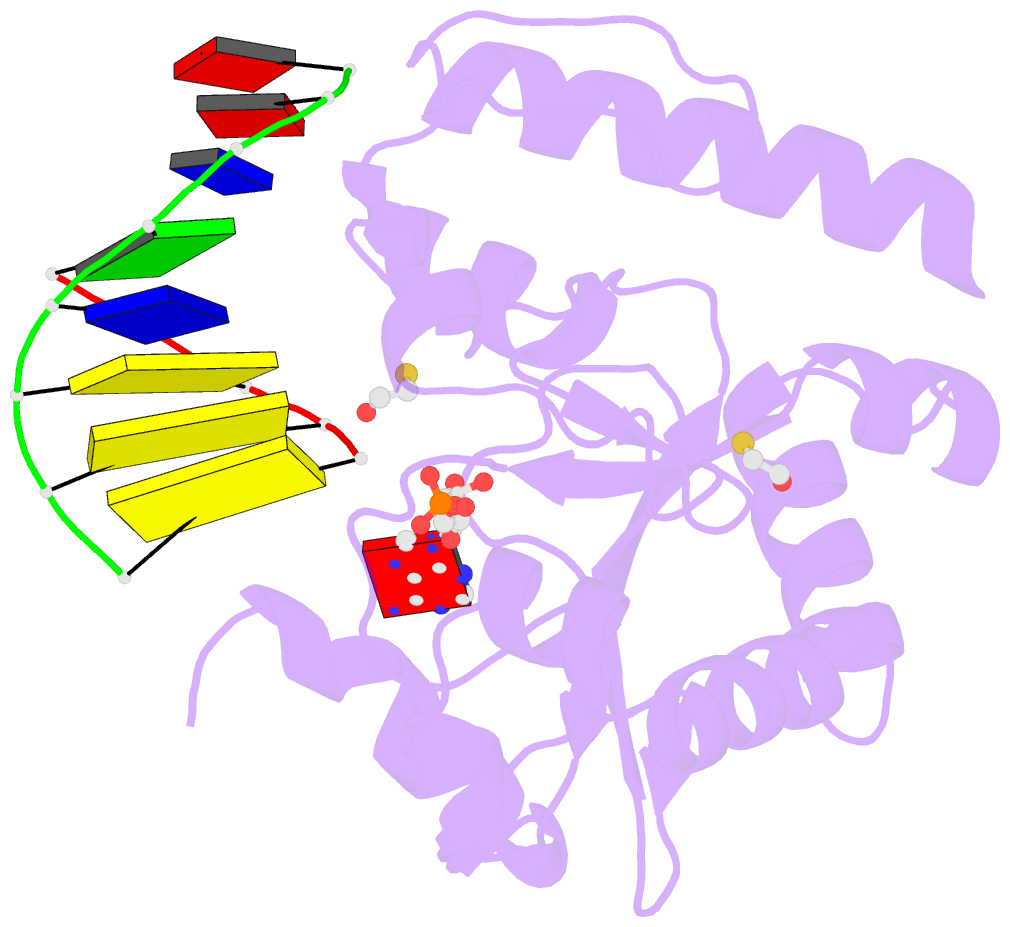
Summary information and primary citation
- PDB-id
-
3szq;
SNAP-derived features in text and
JSON formats
- Class
- hydrolase-DNA
- Method
- X-ray (2.353 Å)
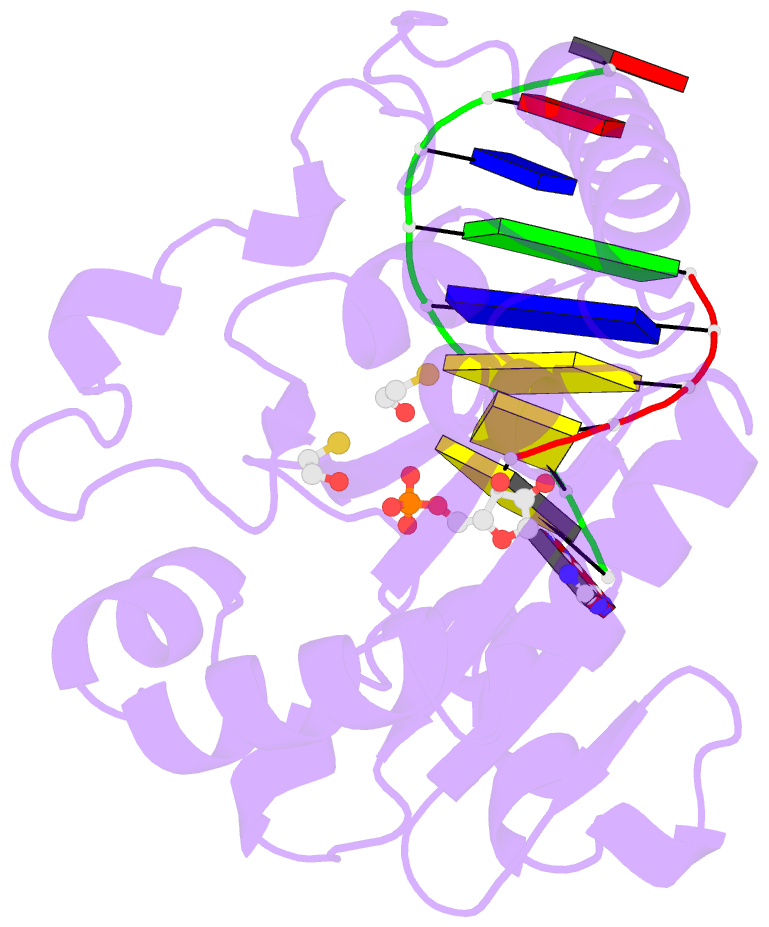
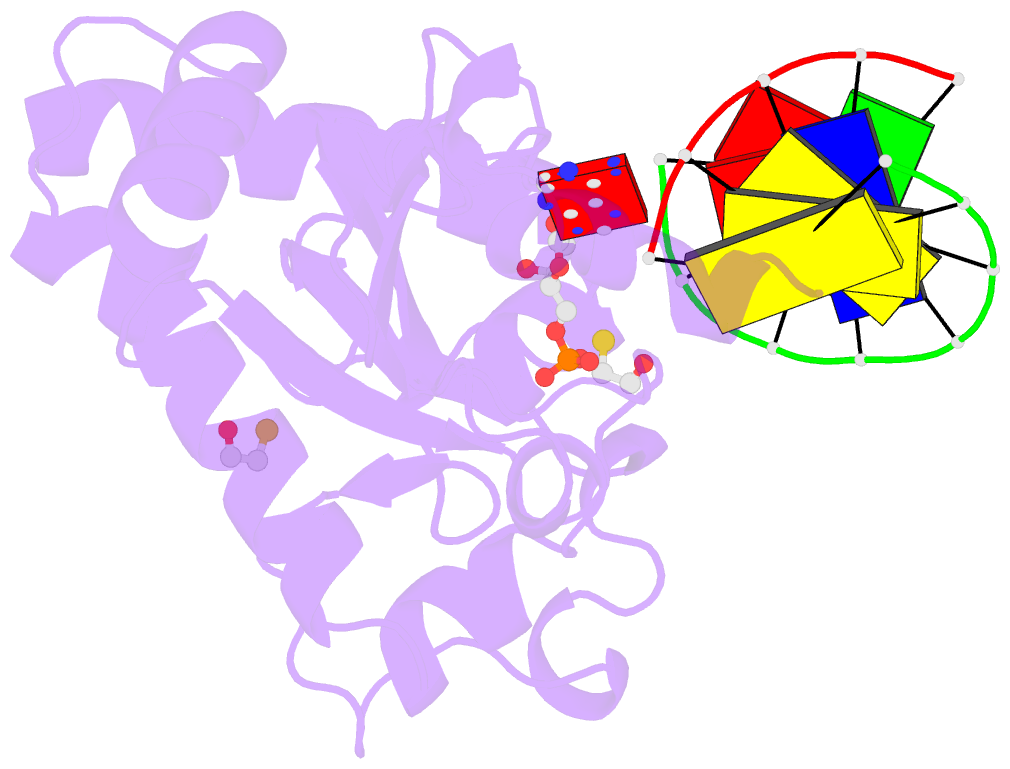
- Summary
- Structure of an s. pombe aptx-DNA-amp-zn complex
- Reference
-
Tumbale P, Appel CD, Kraehenbuehl R, Robertson PD,
Williams JS, Krahn J, Ahel I, Williams RS (2011):
"Structure
of an aprataxin-DNA complex with insights into AOA1
neurodegenerative disease."
Nat.Struct.Mol.Biol., 18,
1189-1195. doi: 10.1038/nsmb.2146.
- Abstract
- DNA ligases finalize DNA replication and repair through
DNA nick-sealing reactions that can abort to generate
cytotoxic 5'-adenylation DNA damage. Aprataxin (Aptx)
catalyzes direct reversal of 5'-adenylate adducts to
protect genome integrity. Here the structure of a
Schizosaccharomyces pombe Aptx-DNA-AMP-Zn(2+) complex
reveals active site and DNA interaction clefts formed by
fusing a histidine triad (HIT) nucleotide hydrolase with a
DNA minor groove-binding C(2)HE zinc finger (Znf). An Aptx
helical 'wedge' interrogates the base stack for sensing DNA
ends or DNA nicks. The HIT-Znf, the wedge and an '[F/Y]PK'
pivot motif cooperate to distort terminal DNA base-pairing
and direct 5'-adenylate into the active site pocket.
Structural and mutational data support a wedge-pivot-cut
HIT-Znf catalytic mechanism for 5'-adenylate adduct
recognition and removal and suggest that mutations
affecting protein folding, the active site pocket and the
pivot motif underlie Aptx dysfunction in the
neurodegenerative disorder ataxia with oculomotor apraxia 1
(AOA1).