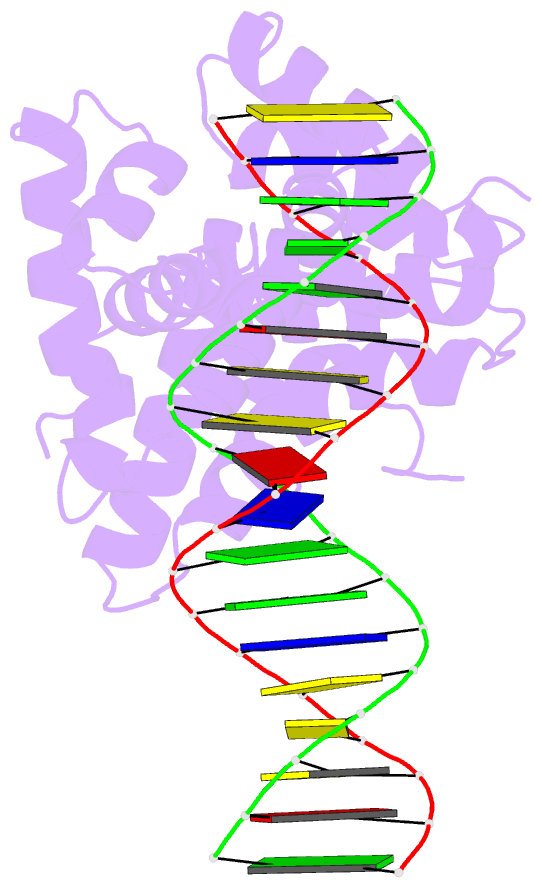
Summary information and primary citation
- PDB-id
- 3mky; DSSR-derived features in text and JSON formats
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.86 Å)
- Summary
- Structure of sopb(155-323)-18mer DNA complex, i23 form
- Reference
- Schumacher MA, Piro KM, Xu W (2010): "Insight into F plasmid DNA segregation revealed by structures of SopB and SopB-DNA complexes." Nucleic Acids Res., 38, 4514-4526. doi: 10.1093/nar/gkq161.
- Abstract
- Accurate DNA segregation is essential for genome transmission. Segregation of the prototypical F plasmid requires the centromere-binding protein SopB, the NTPase SopA and the sopC centromere. SopB displays an intriguing range of DNA-binding properties essential for partition; it binds sopC to form a partition complex, which recruits SopA, and it also coats DNA to prevent non-specific SopA-DNA interactions, which inhibits SopA polymerization. To understand the myriad functions of SopB, we determined a series of SopB-DNA crystal structures. SopB does not distort its DNA site and our data suggest that SopB-sopC forms an extended rather than wrapped partition complex with the SopA-interacting domains aligned on one face. SopB is a multidomain protein, which like P1 ParB contains an all-helical DNA-binding domain that is flexibly attached to a compact (beta(3)-alpha)(2) dimer-domain. Unlike P1 ParB, the SopB dimer-domain does not bind DNA. Moreover, SopB contains a unique secondary dimerization motif that bridges between DNA duplexes. Both specific and non-specific SopB-DNA bridging structures were observed. This DNA-linking function suggests a novel mechanism for in trans DNA spreading by SopB, explaining how it might mask DNA to prevent DNA-mediated inhibition of SopA polymerization.