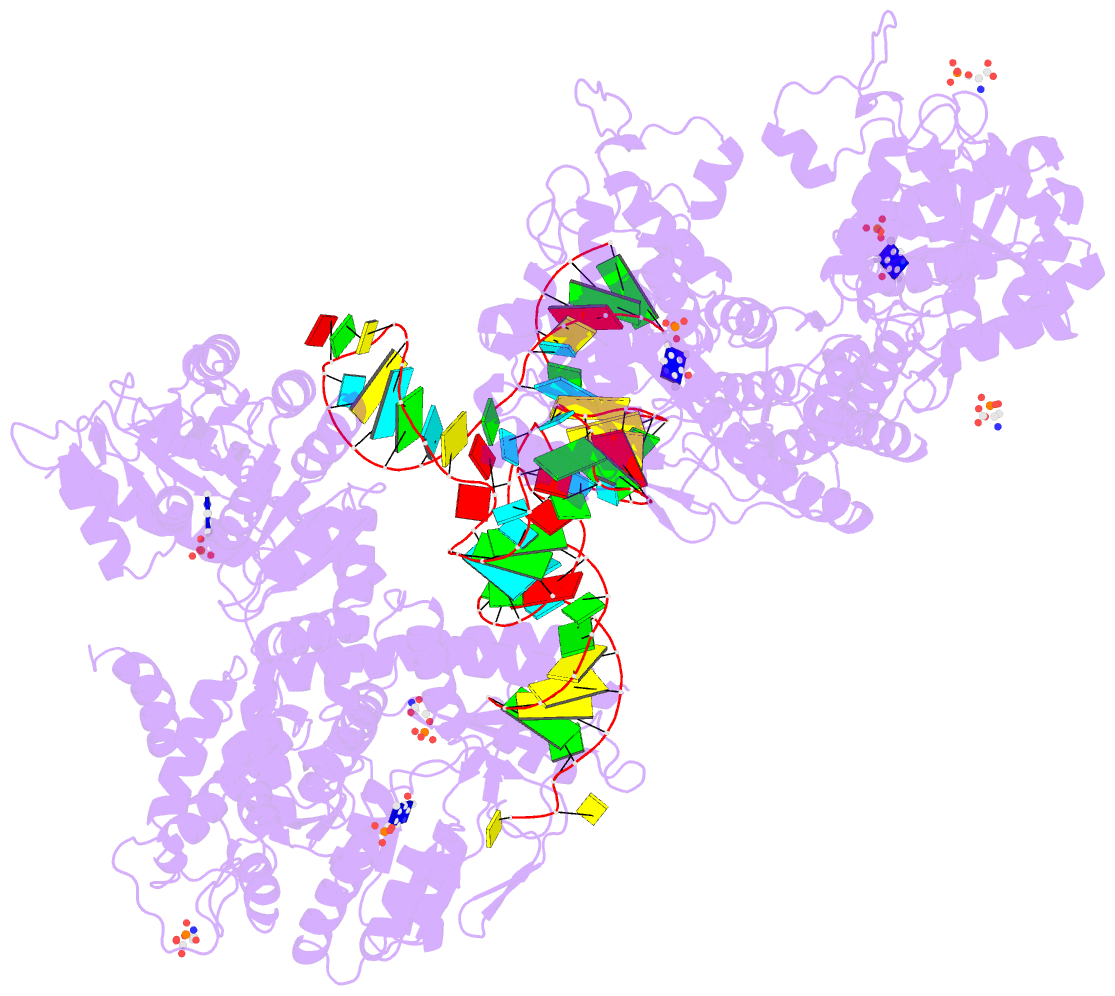
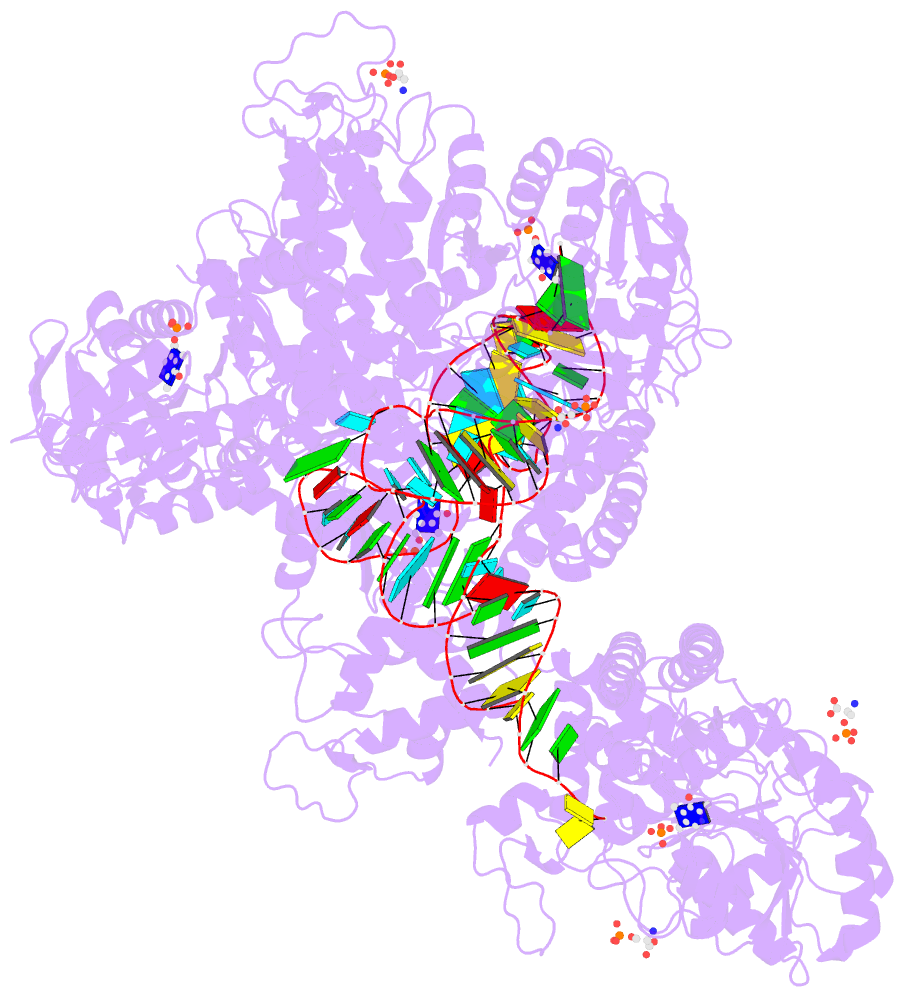
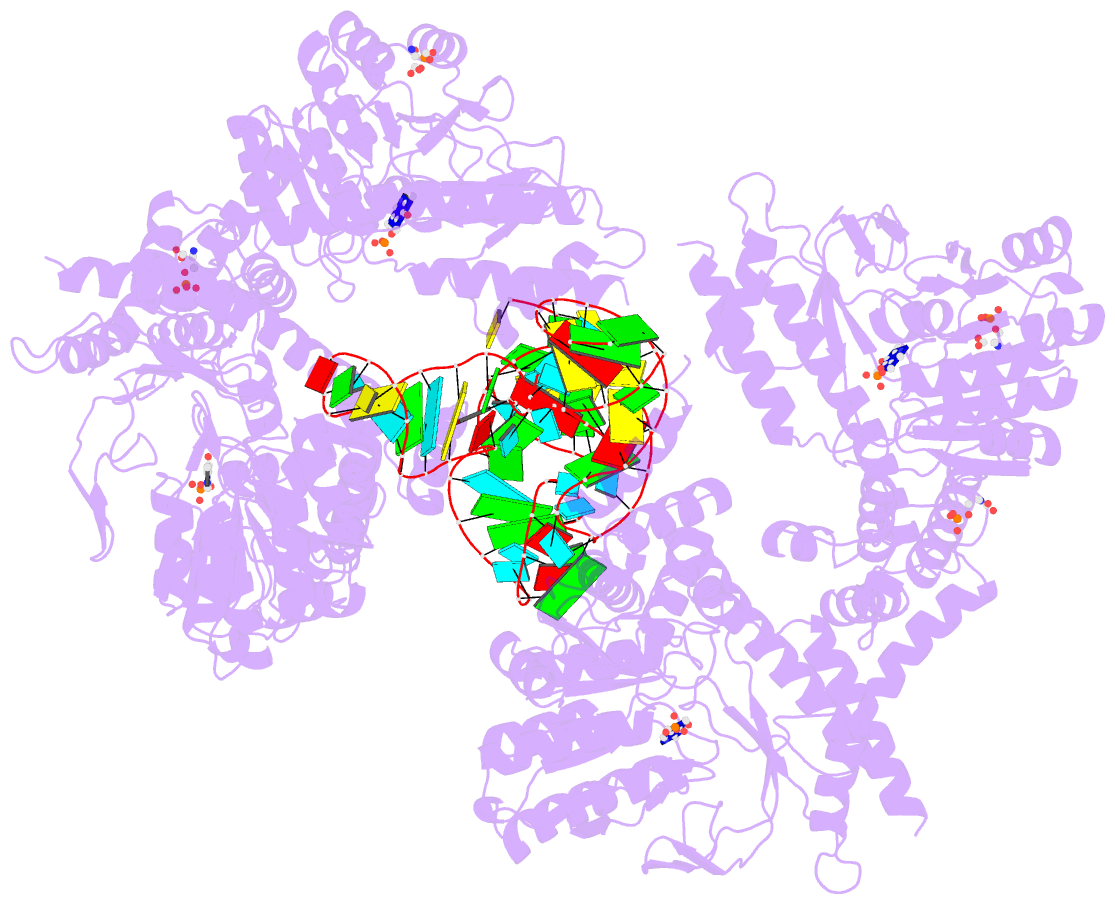
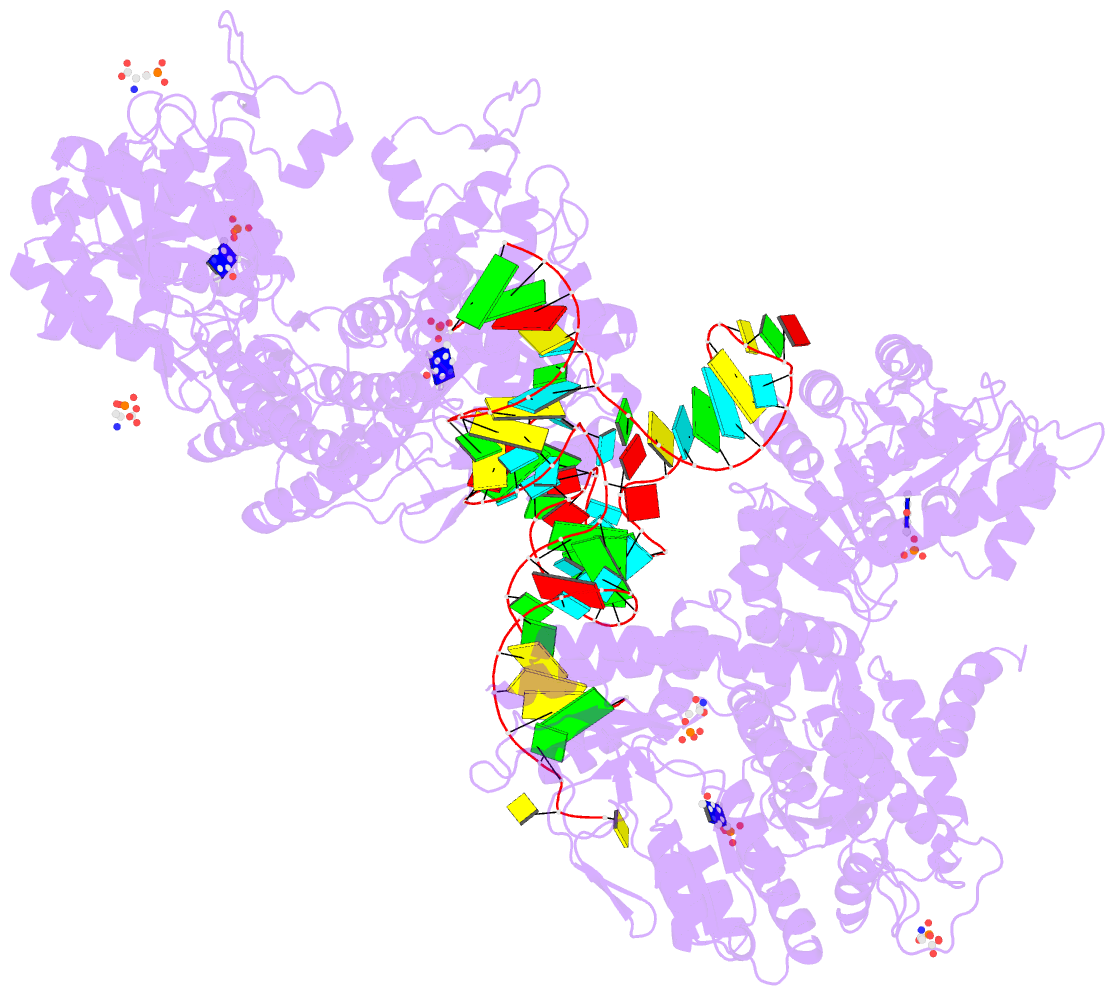
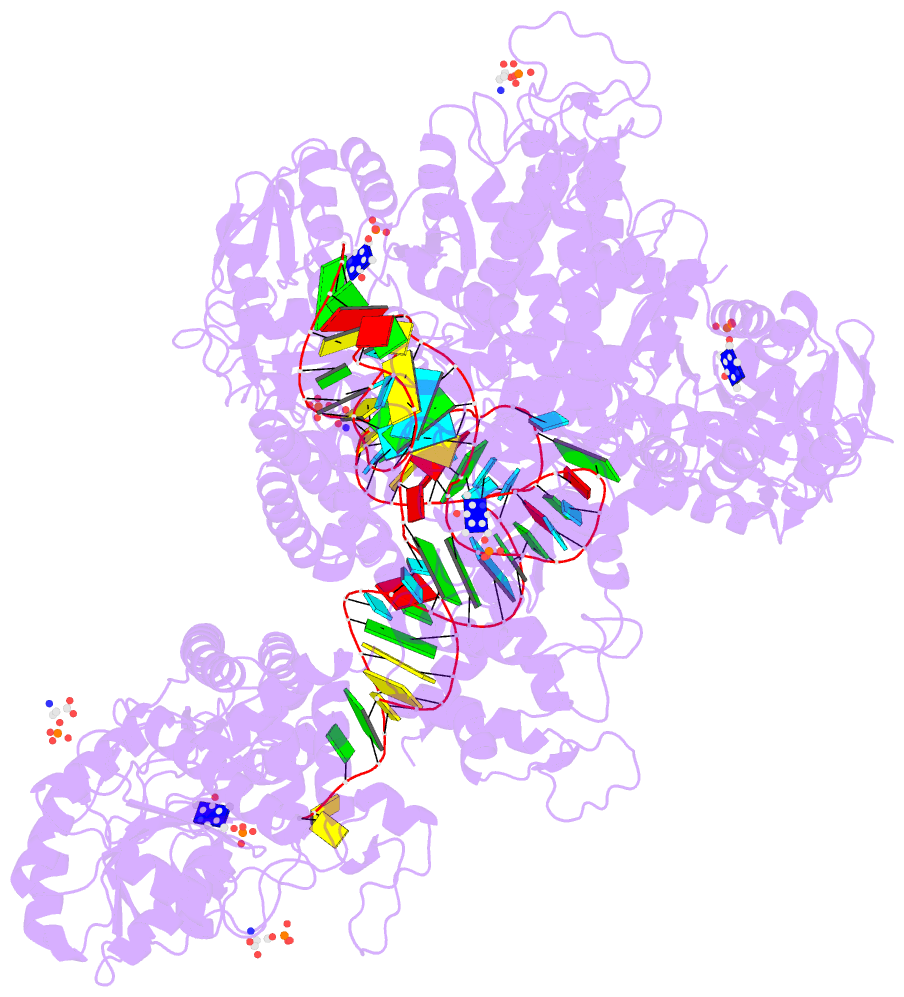
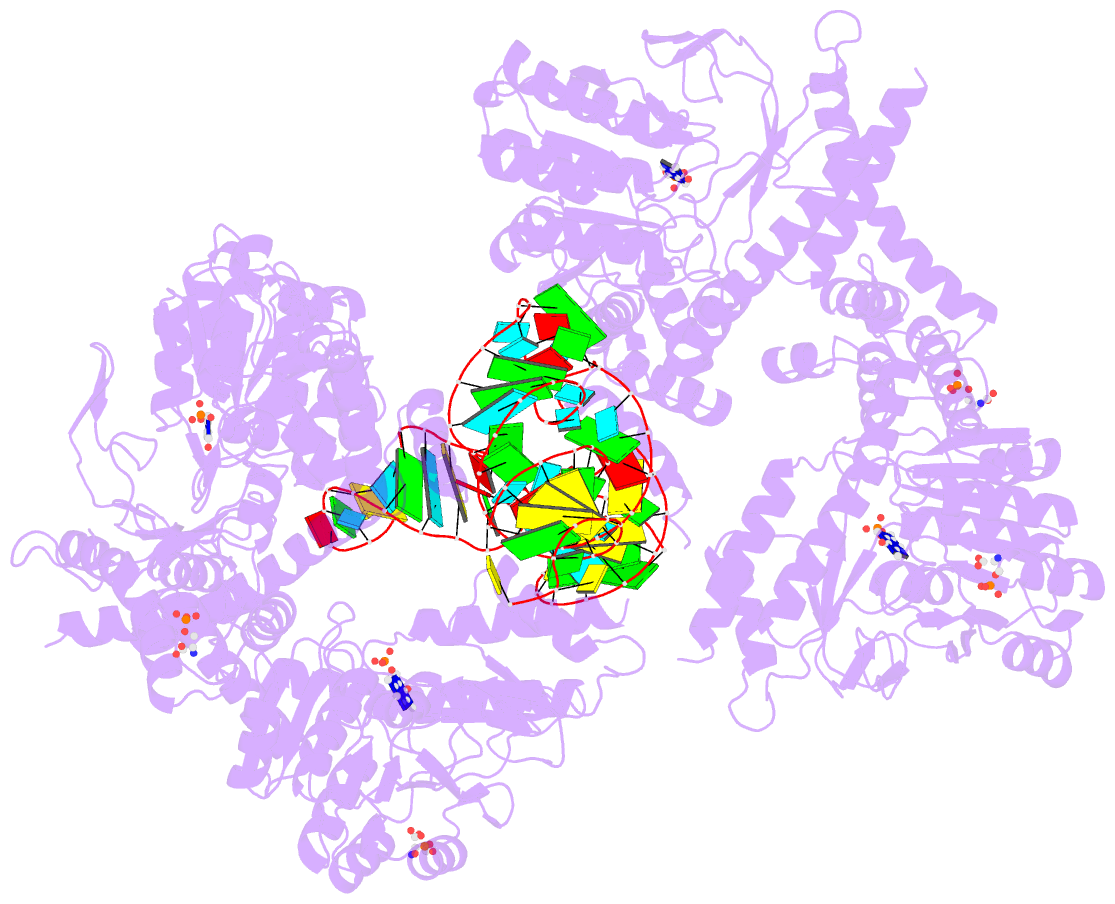
Summary information and primary citation
- PDB-id
- 3hl2; DSSR-derived features in text and JSON formats
- Class
- transferase
- Method
- X-ray (2.81 Å)
- Summary
- The crystal structure of the human sepsecs-trnasec complex
- Reference
- Palioura S, Sherrer RL, Steitz TA, Soll D, Simonovic M (2009): "The human SepSecS-tRNASec complex reveals the mechanism of selenocysteine formation." Science, 325, 321-325. doi: 10.1126/science.1173755.
- Abstract
- Selenocysteine is the only genetically encoded amino acid in humans whose biosynthesis occurs on its cognate transfer RNA (tRNA). O-Phosphoseryl-tRNA:selenocysteinyl-tRNA synthase (SepSecS) catalyzes the final step of selenocysteine formation by a poorly understood tRNA-dependent mechanism. The crystal structure of human tRNA(Sec) in complex with SepSecS, phosphoserine, and thiophosphate, together with in vivo and in vitro enzyme assays, supports a pyridoxal phosphate-dependent mechanism of Sec-tRNA(Sec) formation. Two tRNA(Sec) molecules, with a fold distinct from other canonical tRNAs, bind to each SepSecS tetramer through their 13-base pair acceptor-TPsiC arm (where Psi indicates pseudouridine). The tRNA binding is likely to induce a conformational change in the enzyme's active site that allows a phosphoserine covalently attached to tRNA(Sec), but not free phosphoserine, to be oriented properly for the reaction to occur.