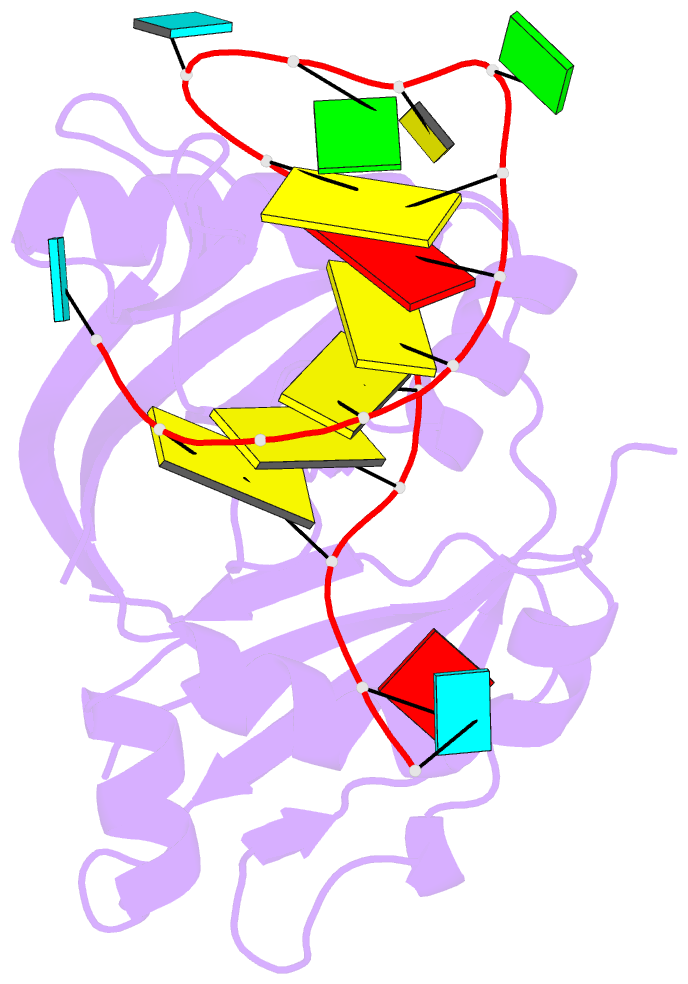
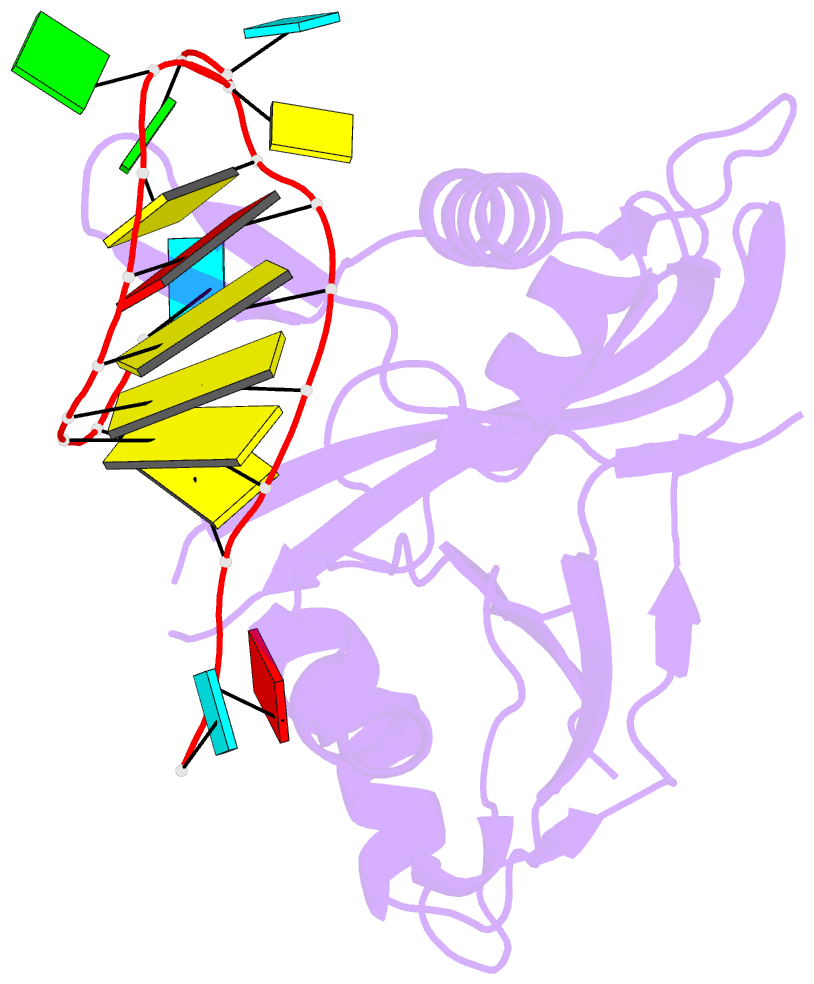
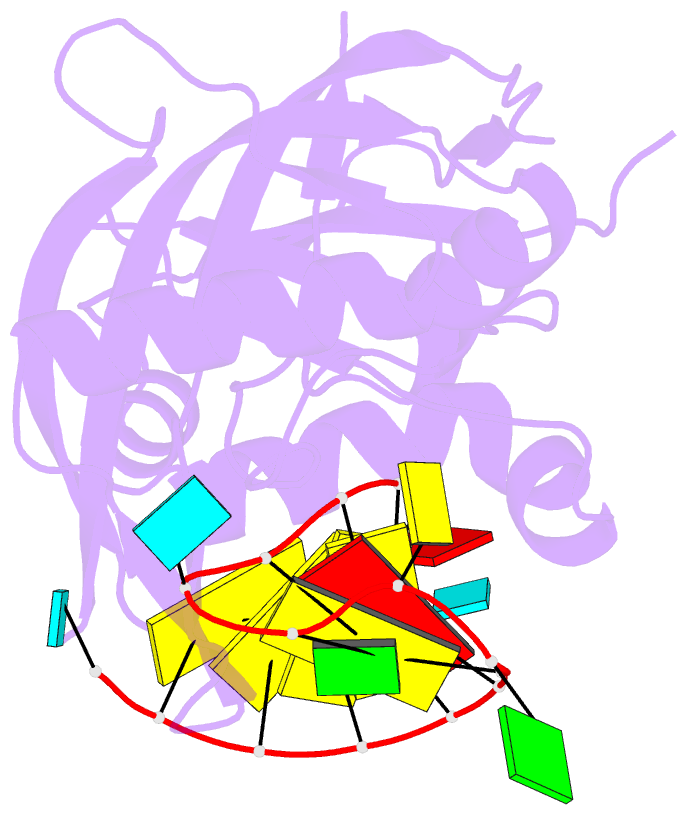
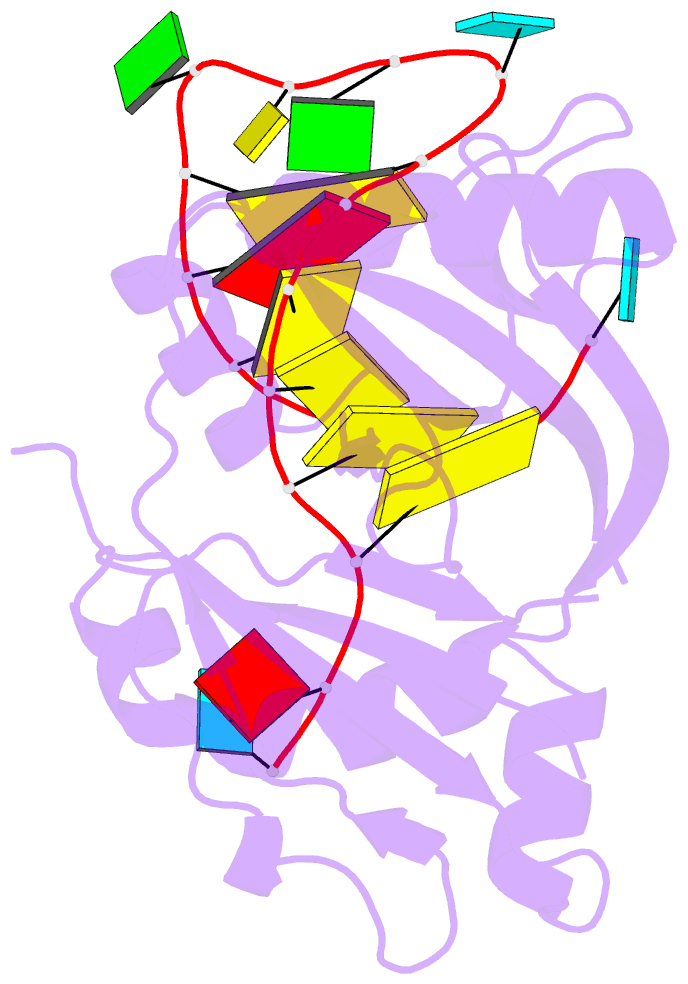
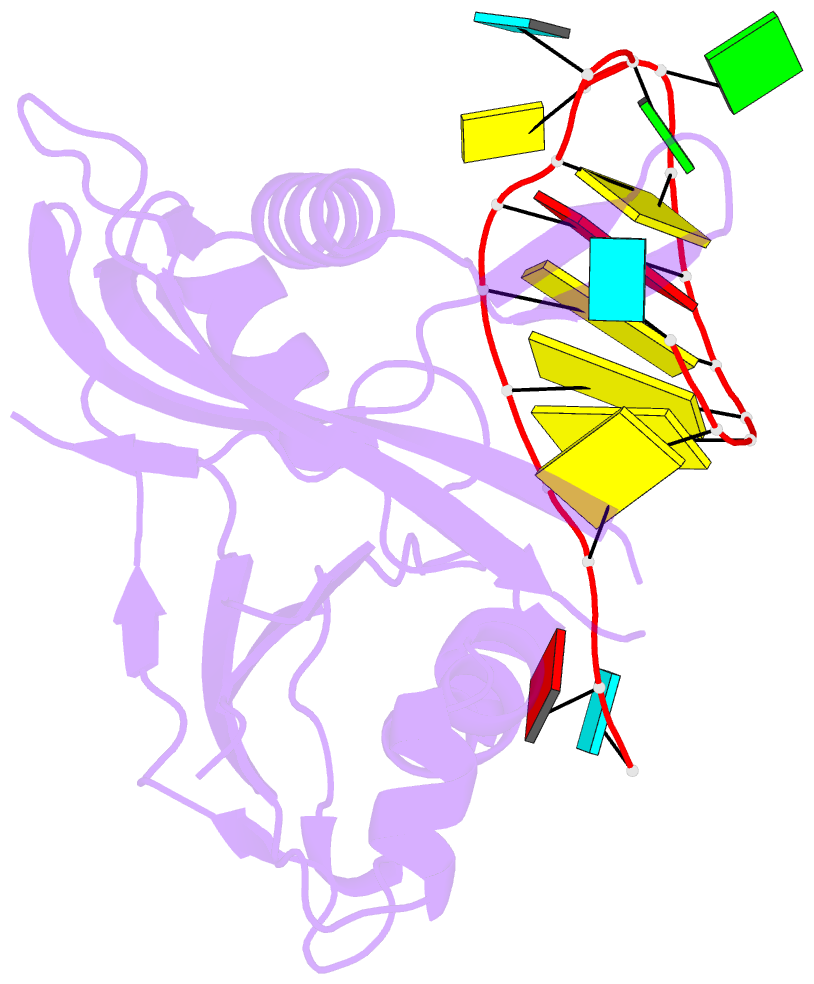
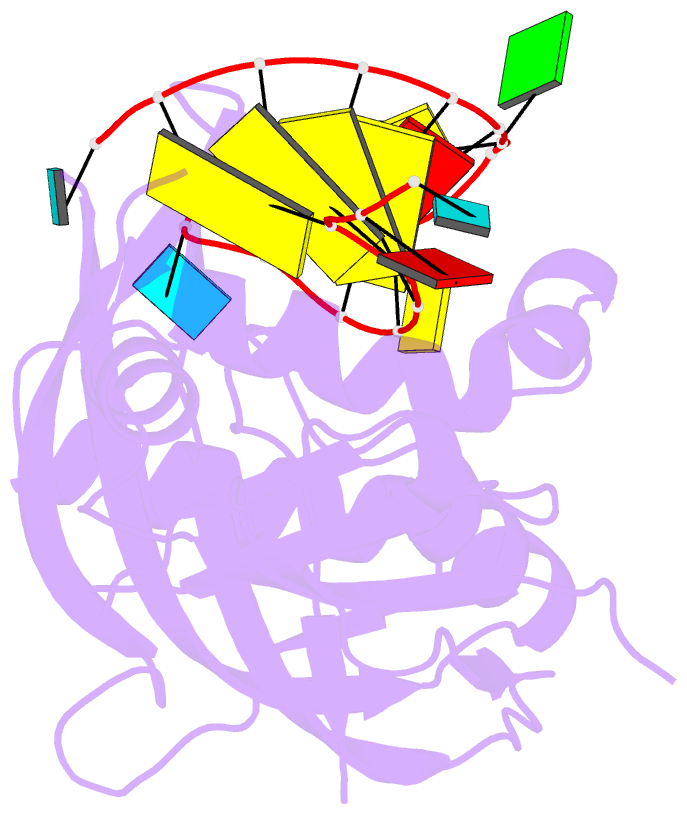
Summary information and primary citation
- PDB-id
- 2y8y; DSSR-derived features in text and JSON formats
- Class
- hydrolase-RNA
- Method
- X-ray (1.44 Å)
- Summary
- Structure b of crispr endoribonuclease cse3 bound to 19 nt RNA
- Reference
- Sashital DG, Jinek M, Doudna JA (2011): "An RNA-Induced Conformational Change Required for Crispr RNA Cleavage by the Endoribonuclease Cse3." Nat.Struct.Mol.Biol., 18, 680. doi: 10.1038/NSMB.2043.
- Abstract
- Clustered regularly interspaced short palindromic repeat (CRISPR) chromosomal loci found in prokaryotes provide an adaptive immune system against bacteriophages and plasmids. CRISPR-specific endoRNases produce short RNA molecules (crRNAs) from CRISPR transcripts, which harbor sequences complementary to invasive nucleic acid elements and ensure their selective targeting by CRISPR-associated (Cas) proteins. The extreme sequence divergence of CRISPR-specific endoRNases and their RNA substrates has obscured homology-based comparison of RNA recognition and cleavage mechanisms. Here, we show that Cse3 type CRISPR-specific endoRNases bind a hairpin structure and residues downstream of the cleavage site within the repetitive segment of cognate CRISPR RNA. Cocrystal structures of Cse3-RNA complexes reveal an RNA-induced conformational change in the enzyme active site that aligns the RNA strand for site-specific cleavage. These studies provide insight into a catalytically essential RNA recognition mechanism by a large class of CRISPR-related endoRNases.