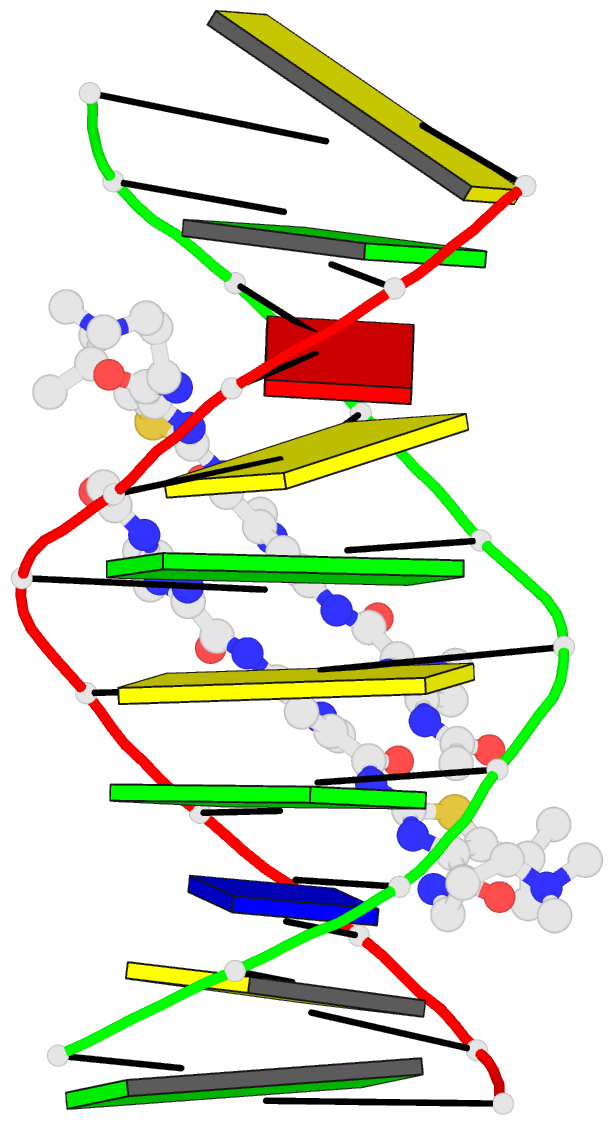
Summary information and primary citation
- PDB-id
- 2mnd; DSSR-derived features in text and JSON formats
- Class
- DNA
- Method
- NMR
- Summary
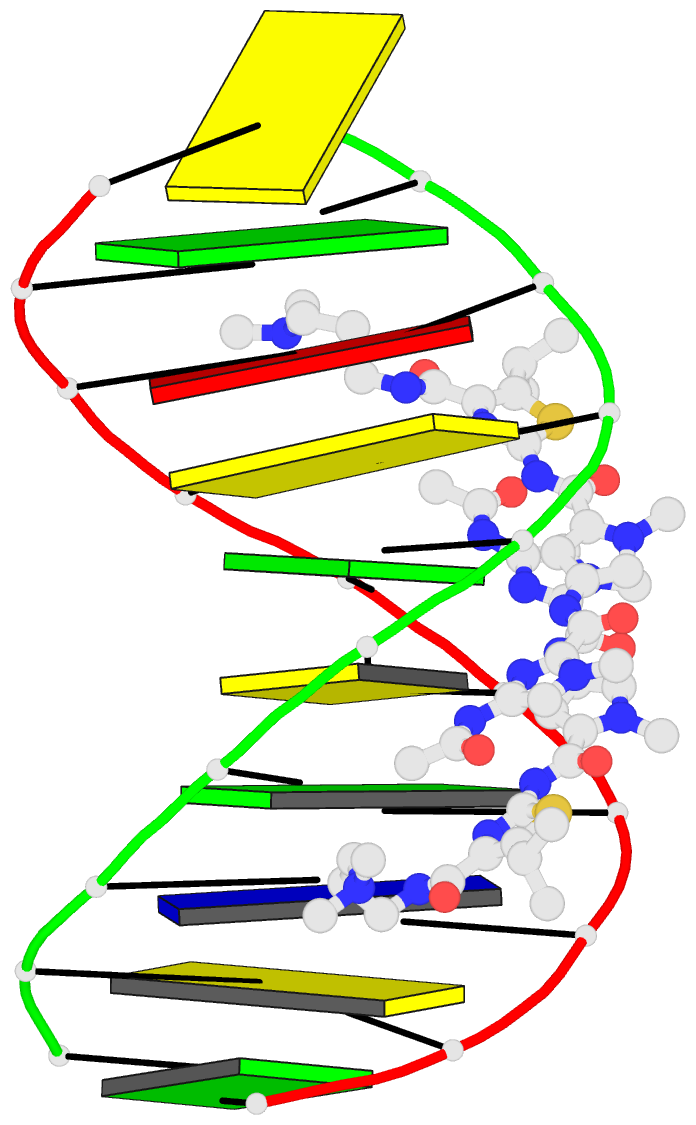
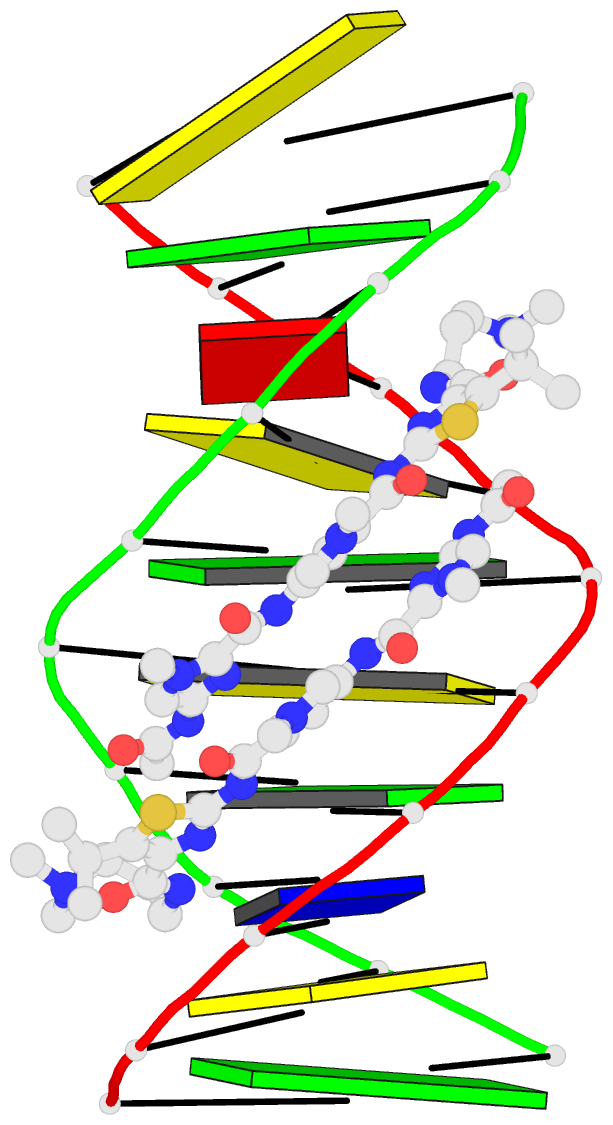
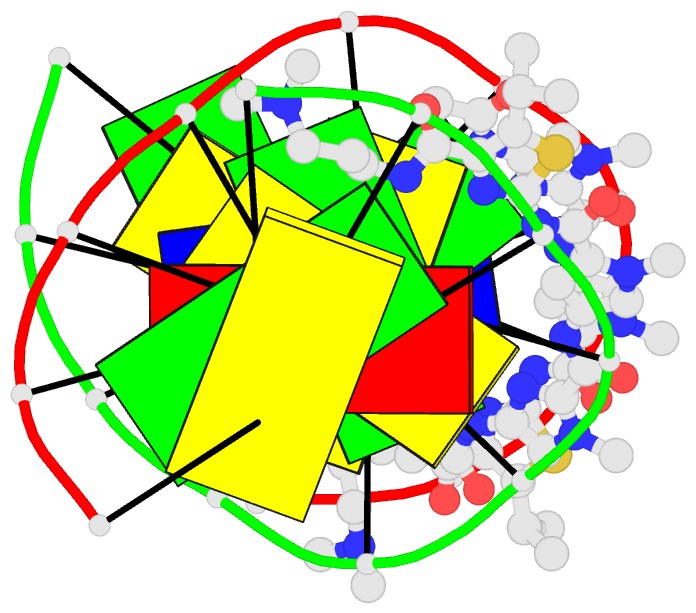
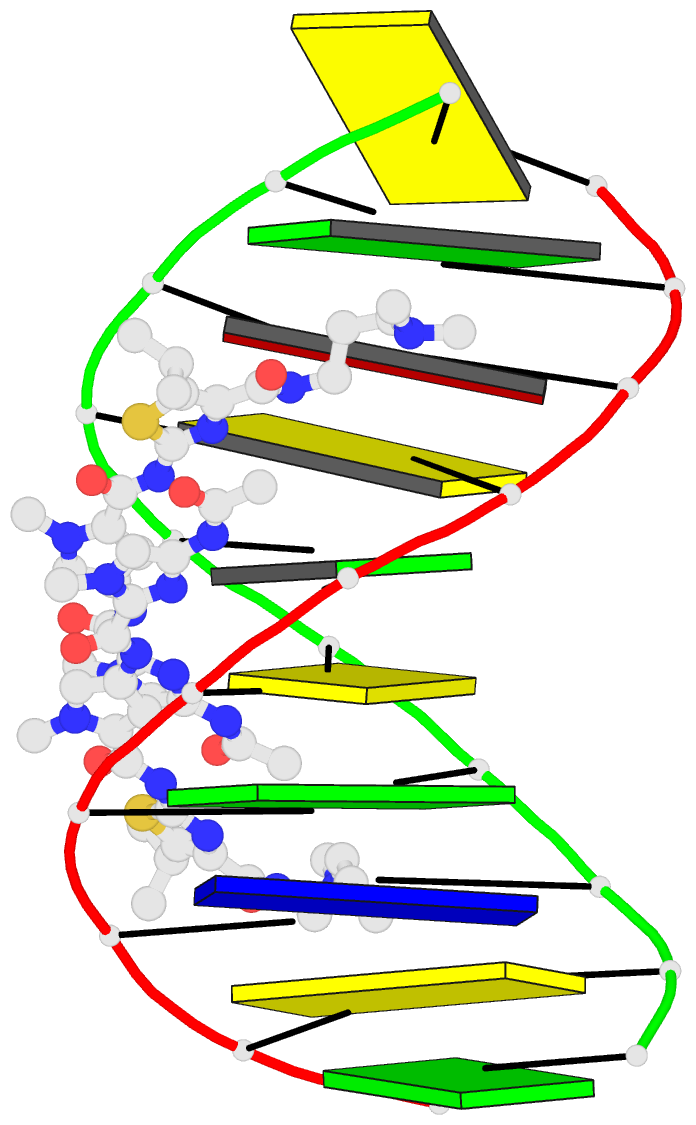
- Recognition complex of DNA d(cgacgcgtcg)2 with thiazotropsin b
- Reference
- Alniss HY, Salvia MV, Sadikov M, Golovchenko I, Anthony NG, Khalaf AI, MacKay SP, Suckling CJ, Parkinson JA (2014): "Recognition of the DNA minor groove by thiazotropsin analogues." Chembiochem, 15, 1978-1990. doi: 10.1002/cbic.201402202.
- Abstract
- Solution-phase self-association characteristics and DNA molecular-recognition properties are reported for three close analogues of minor-groove-binding ligands from the thiazotropsin class of lexitropsin molecules; they incorporate isopropyl thiazole as a lipophilic building block. Thiazotropsin B (AcImPy(iPr) ThDp) shows similar self-assembly characteristics to thiazotropsin A (FoPyPy(iPr) ThDp), although it is engineered, by incorporation of imidazole in place of N-methyl pyrrole, to swap its DNA recognition target from 5'-ACTAGT-3' to 5'-ACGCGT-3'. Replacement of the formamide head group in thiazotropsin A by nicotinamide in AIK-18/51 results in a measureable difference in solution-phase self-assembly character and substantially enhanced DNA association characteristics. The structures and associated thermodynamic parameters of self-assembled ligand aggregates and their complexes with their respective DNA targets are considered in the context of cluster targeting of DNA by minor-groove complexes.