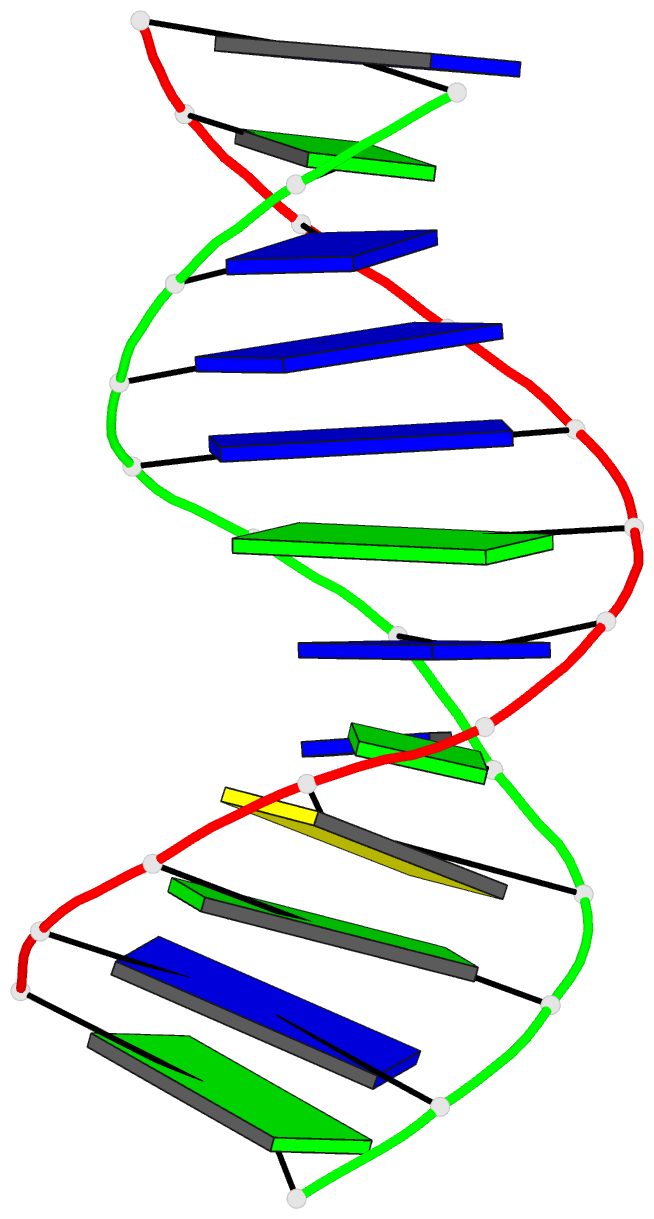
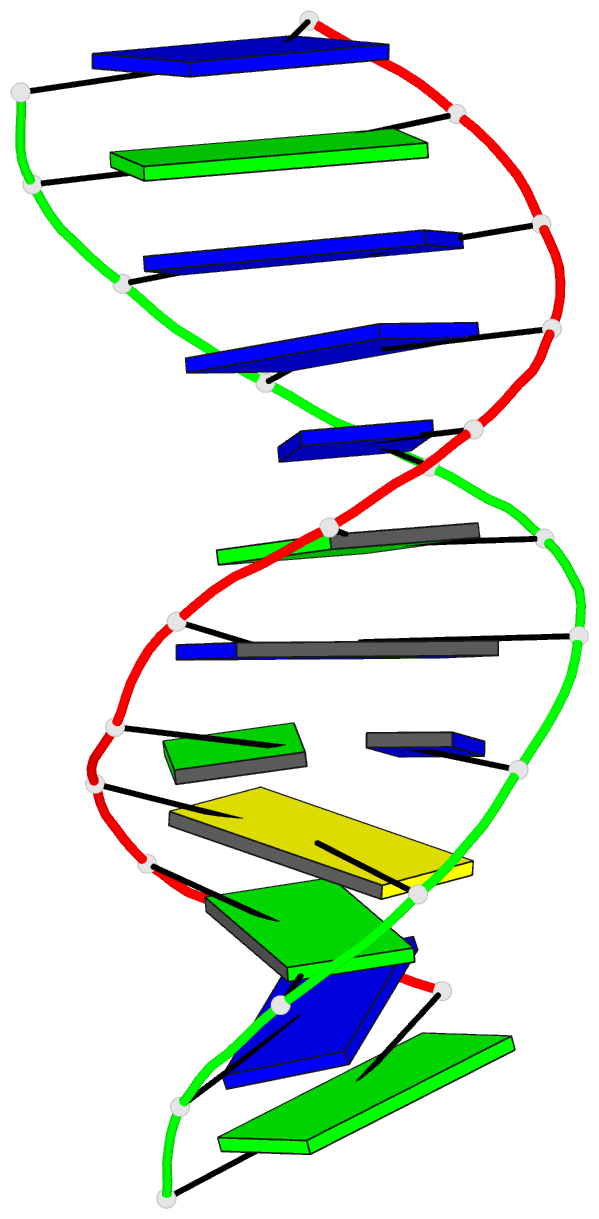
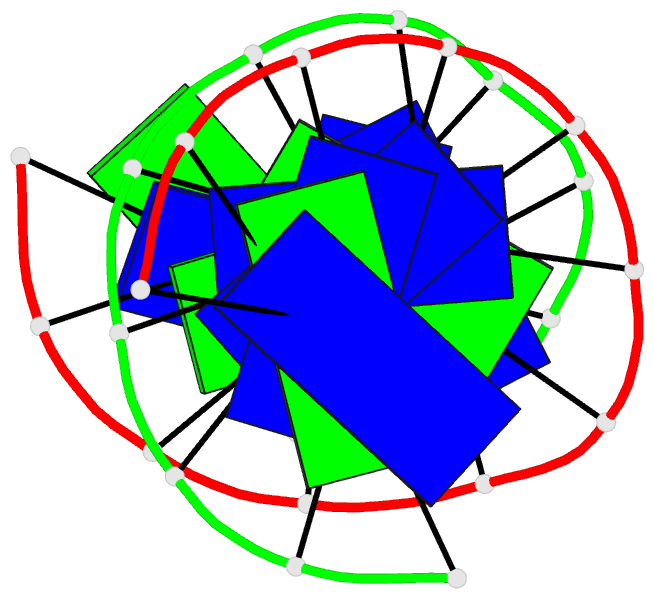
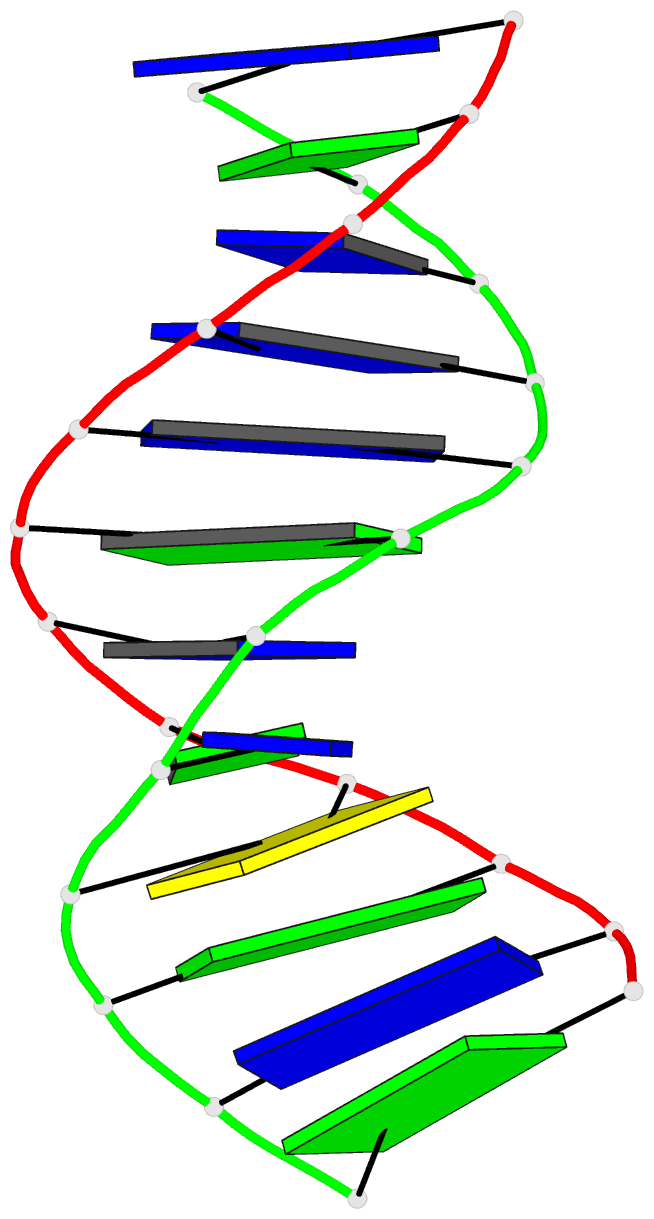
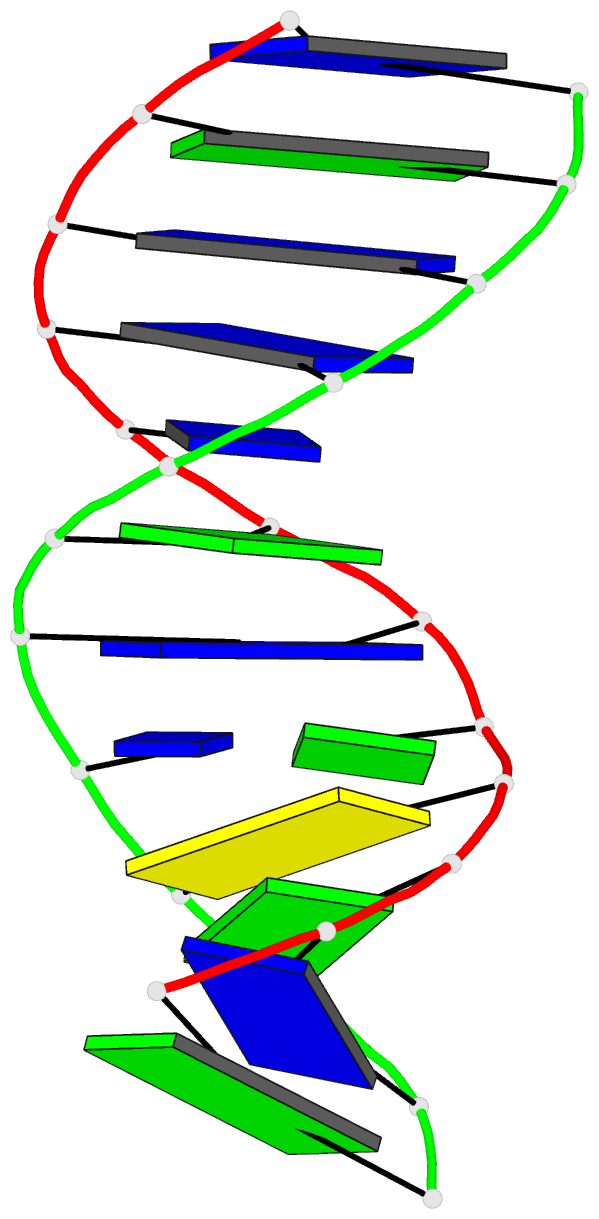
Summary information and primary citation
- PDB-id
- 2lfx; DSSR-derived features in text and JSON formats
- Class
- DNA
- Method
- NMR
- Summary
- Structure of the duplex when (5's)-8,5'-cyclo-2'-deoxyguanosine is placed opposite dt
- Reference
- Huang H, Das RS, Basu AK, Stone MP (2012): "Structures of (5'S)-8,5'-Cyclo-2'-deoxyguanosine Mismatched with dA or dT." Chem.Res.Toxicol., 25, 478-490. doi: 10.1021/tx2005053.
- Abstract
- Diastereomeric 8,5'-cyclopurine 2'-deoxynucleosides, containing a covalent bond between the deoxyribose and the purine base, are induced in DNA by ionizing radiation. They are suspected to play a role in the etiology of neurodegeneration in xeroderma pigmentosum patients. If not repaired, the S-8,5'-cyclo-2'-deoxyguanosine lesion (S-cdG) induces Pol V-dependent mutations at a frequency of 34% in Escherichia coli. Most are S-cdG → A transitions, suggesting mis-incorporation of dTTP opposite the lesion during replication bypass, although low levels of S-cdG → T transversions, arising from mis-incorporation of dATP, are also observed. We report the structures of 5'-d(GTGCXTGTTTGT)-3'·5'-d(ACAAACAYGCAC)-3', where X denotes S-cdG and Y denotes either dA or dT, corresponding to the situation following mis-insertion of either dTTP or dATP opposite the S-cdG lesion. The S-cdG·dT mismatch pair adopts a wobble base pairing. This provides a plausible rationale for the S-cdG → A transitions. The S-cdG·dA mismatch pair differs in conformation from the dG·dA mismatch pair. For the S-cdG·dA mismatch pair, both S-cdG and dA intercalate, but no hydrogen bonding is observed between S-cdG and dA. This is consistent with the lower levels of S-cdG → T transitions in E. coli.