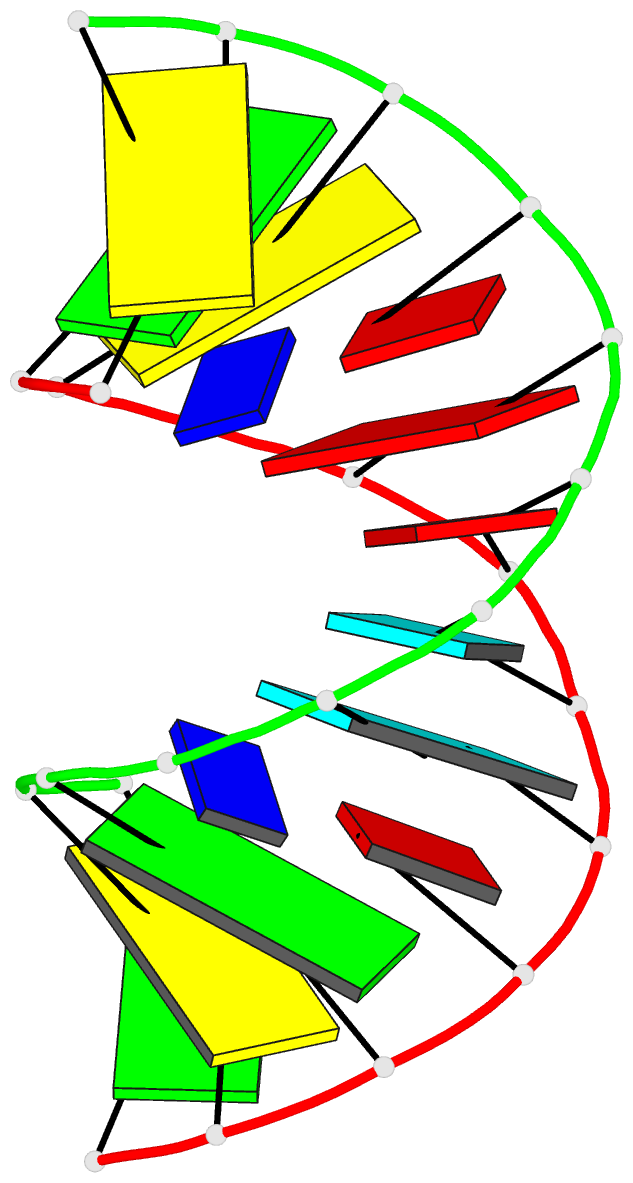
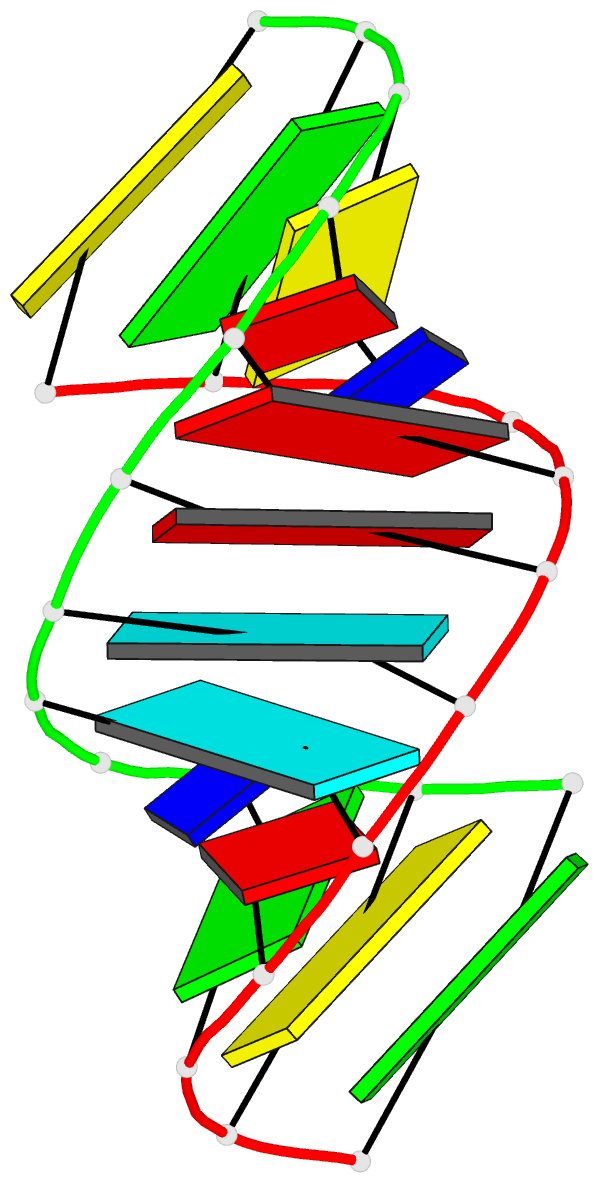
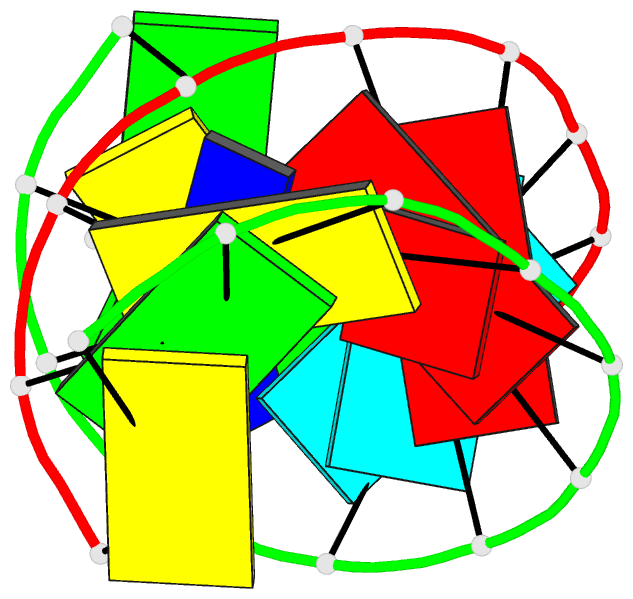
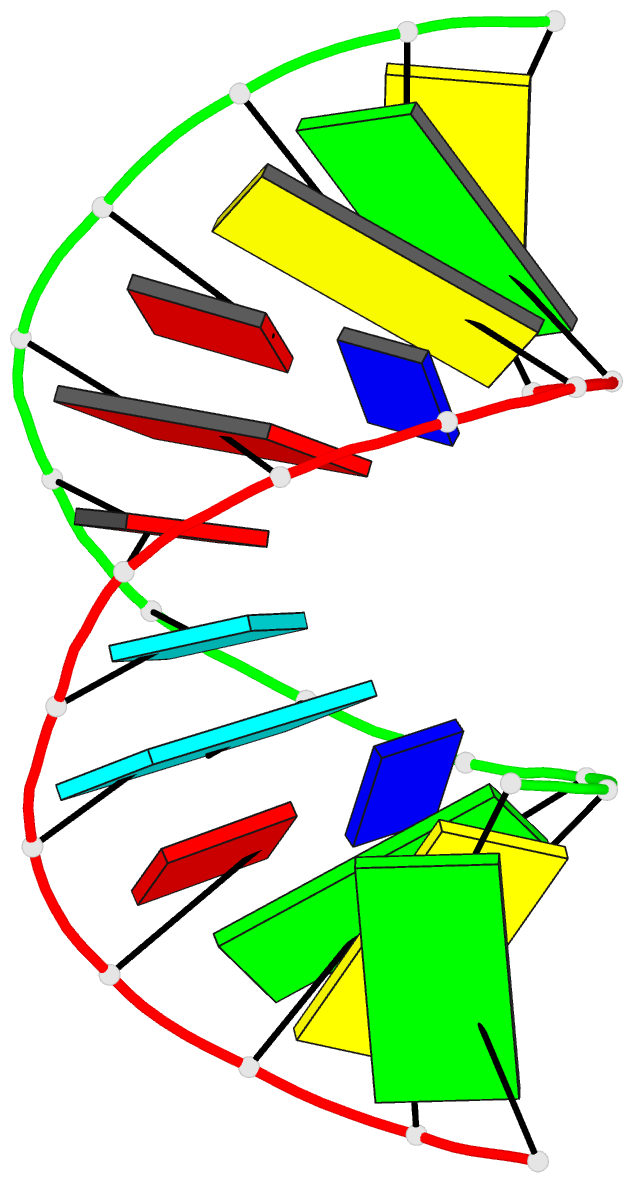
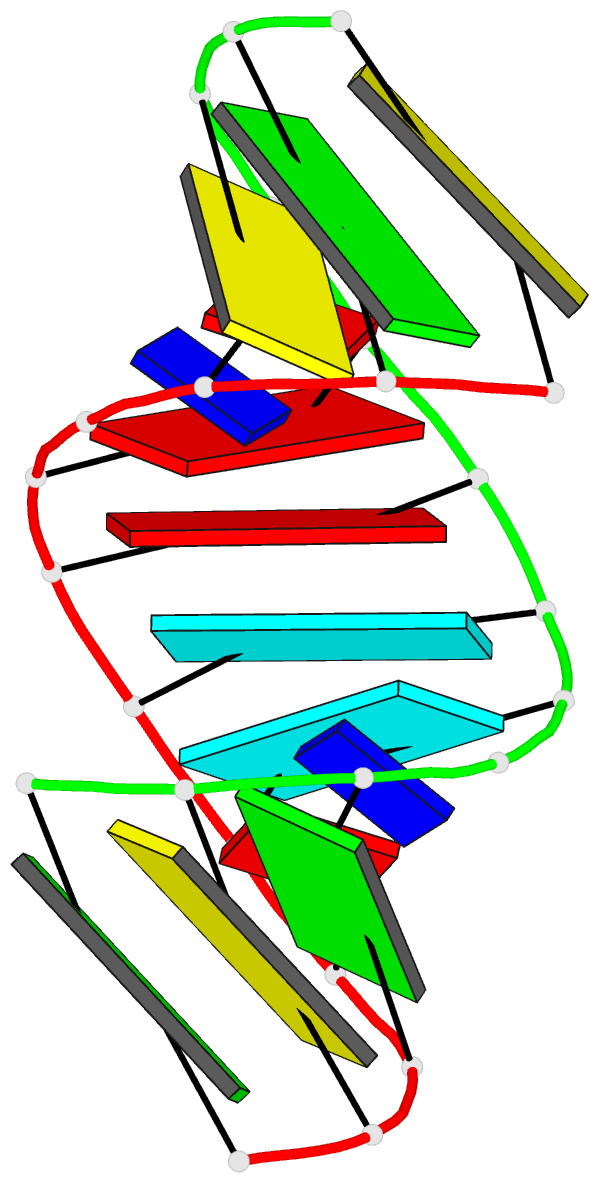
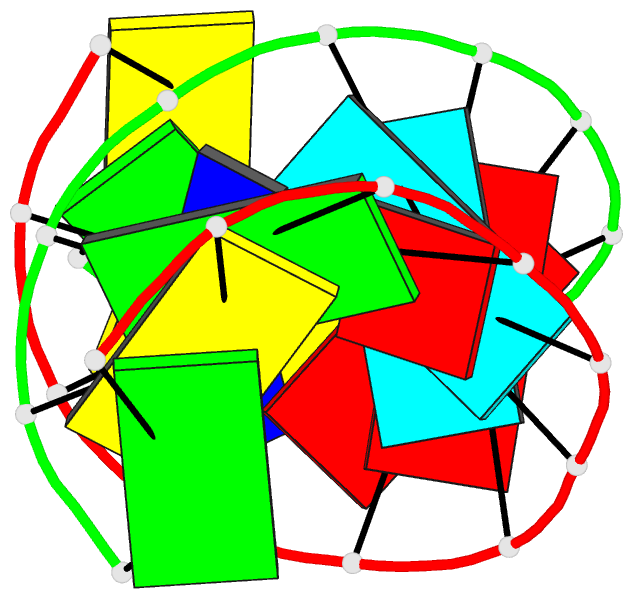
Summary information and primary citation
- PDB-id
- 2g92; DSSR-derived features in text and JSON formats
- Class
- RNA
- Method
- X-ray (1.61 Å)
- Summary
- Crystal structure analysis of the RNA dodecamer cgc-(nf2)-aauuagcg, with an incorporated 2,4-difluorotoluyl residue (nf2)
- Reference
- Xia J, Noronha A, Toudjarska I, Li F, Akinc A, Braich R, Frank-Kamenetsky M, Rajeev KG, Egli M, Manoharan M (2006): "Gene silencing activity of siRNAs with a ribo-difluorotoluyl nucleotide." Acs Chem.Biol., 1, 176-183. doi: 10.1021/cb600063p.
- Abstract
- Recently, chemically synthesized short interfering RNA (siRNA) duplexes have been used with success for gene silencing. Chemical modification is desired for therapeutic applications to improve biostability and pharmacokinetic properties; chemical modification may also provide insight into the mechanism of silencing. siRNA duplexes containing the 2,4-difluorotoluyl ribonucleoside (rF) were synthesized to evaluate the effect of noncanonical nucleoside mimetics on RNA interference. 5'-Modification of the guide strand with rF did not alter silencing relative to unmodified control. Internal uridine to rF substitutions were well-tolerated. Thermal melting analysis showed that the base pair between rF and adenosine (A) was destabilizing relative to a uridine-adenosine pair, although it was slightly less destabilizing than other mismatches. The crystal structure of a duplex containing rFoA pairs showed local structural variations relative to a canonical RNA helix. As the fluorine atoms cannot act as hydrogen bond acceptors and are more hydrophobic than uridine, there was an absence of a well-ordered water structure around the rF residues in both grooves. siRNAs with the rF modification effectively silenced gene expression and offered improved nuclease resistance in serum; therefore, evaluation of this modification in therapeutic siRNAs is warranted.