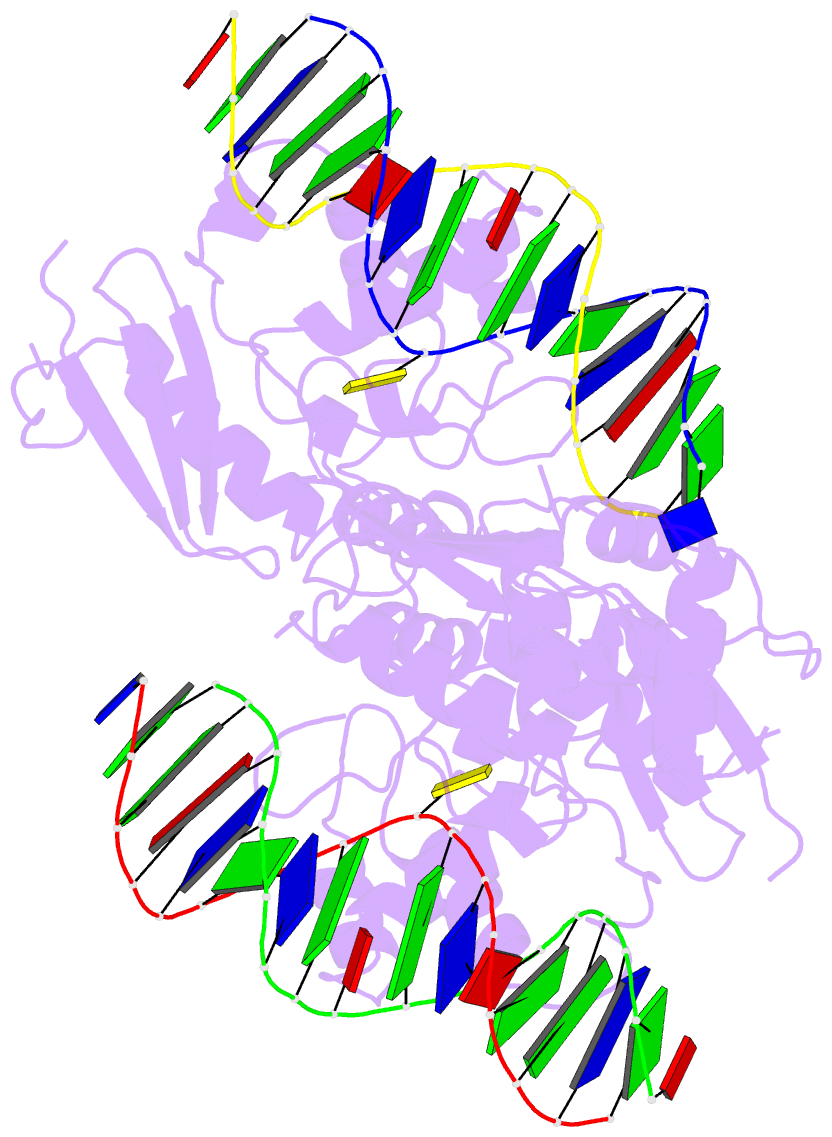
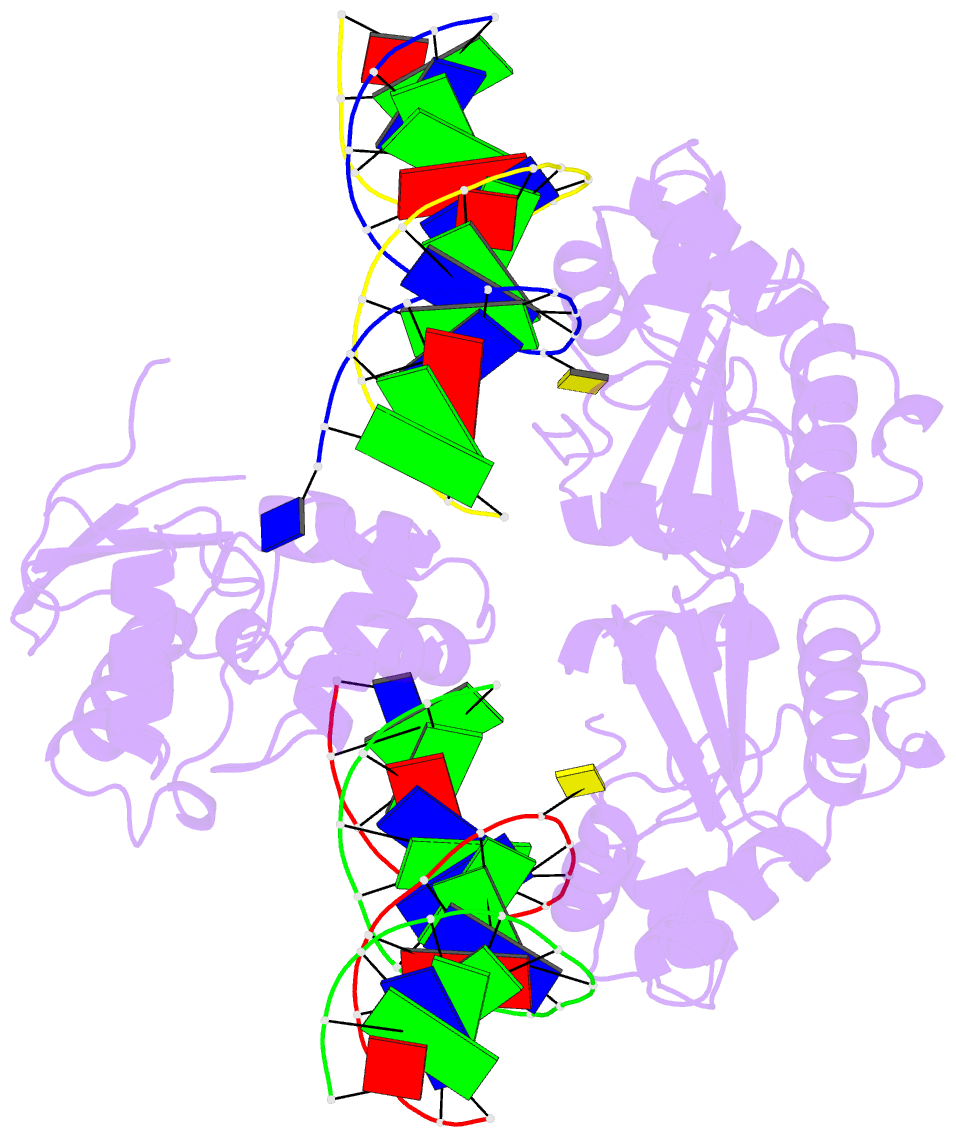
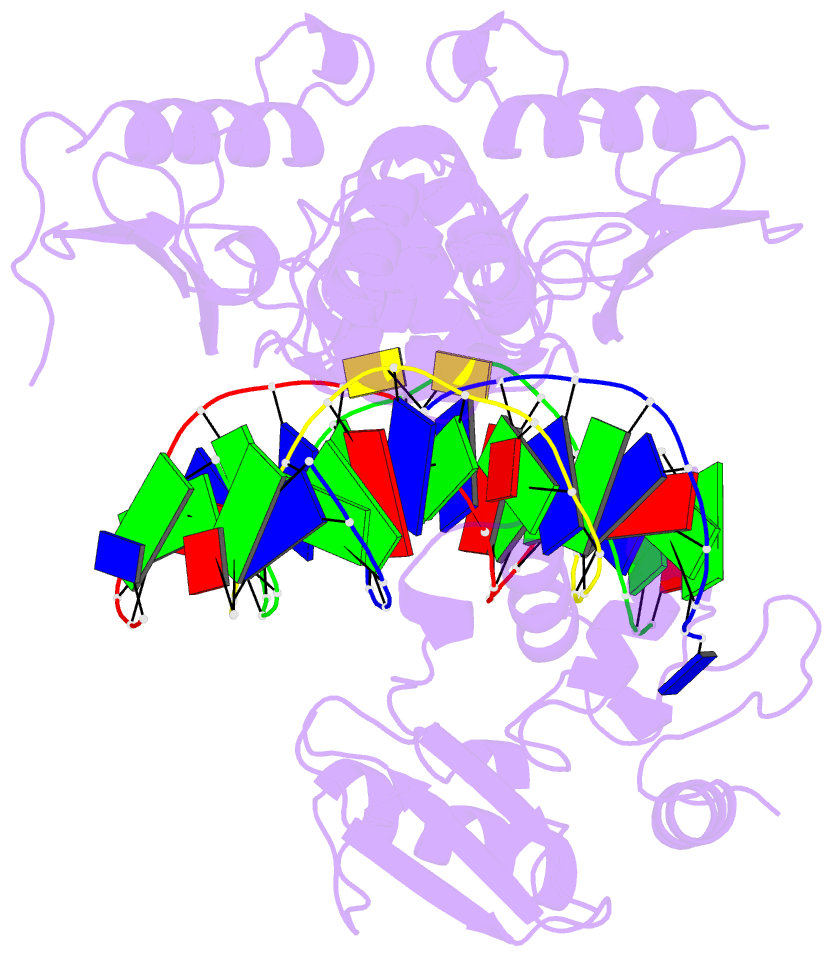
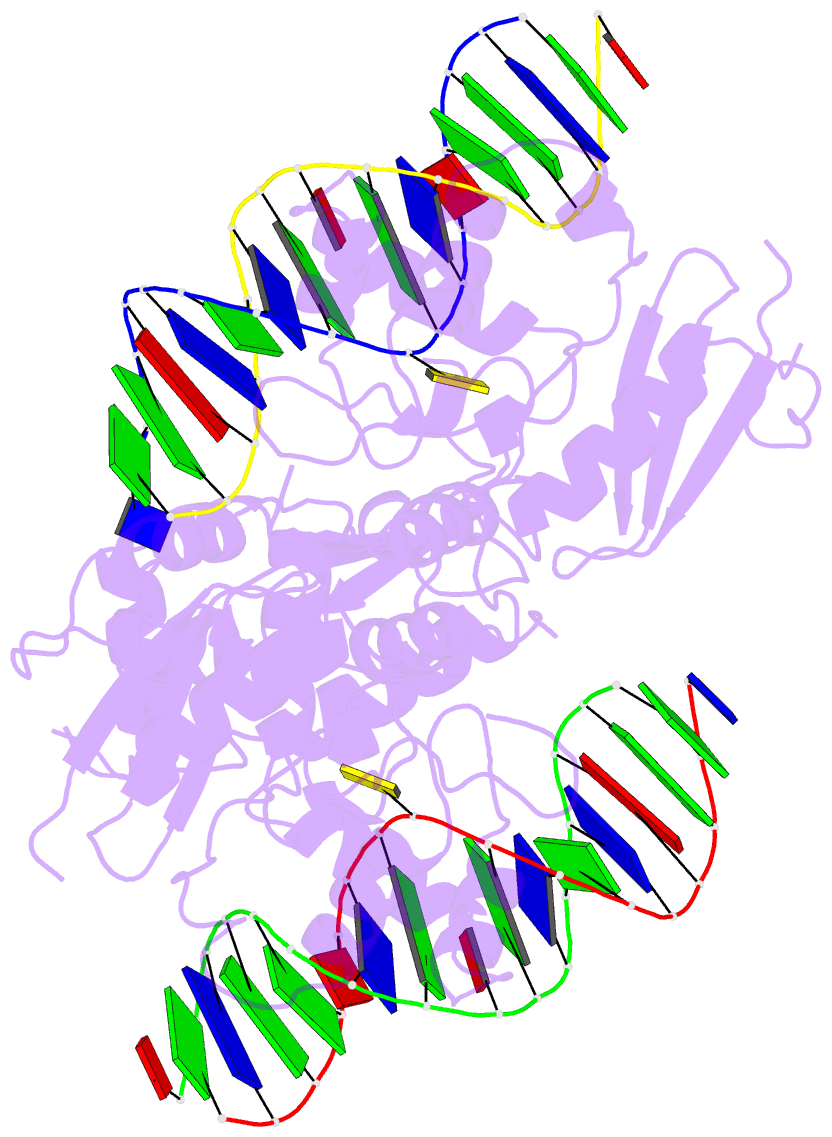
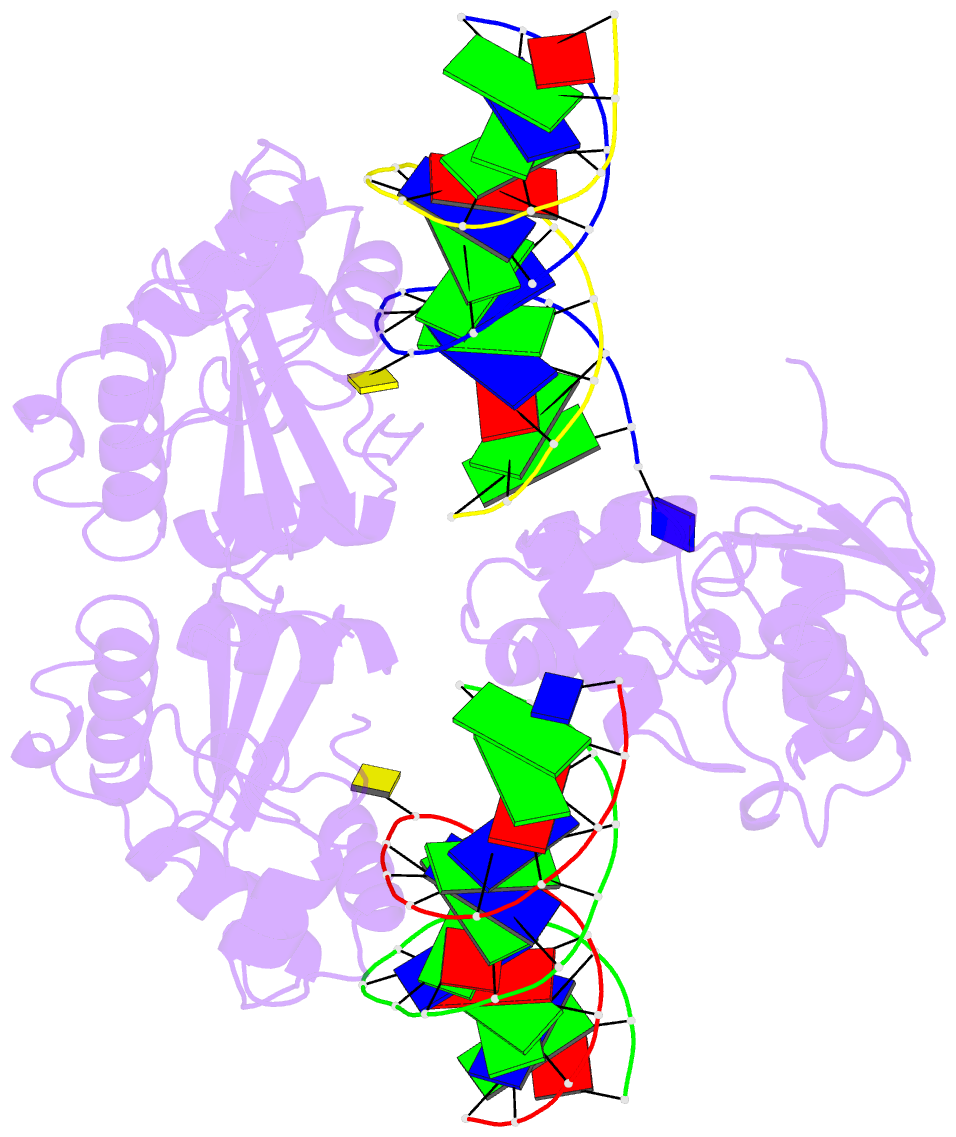
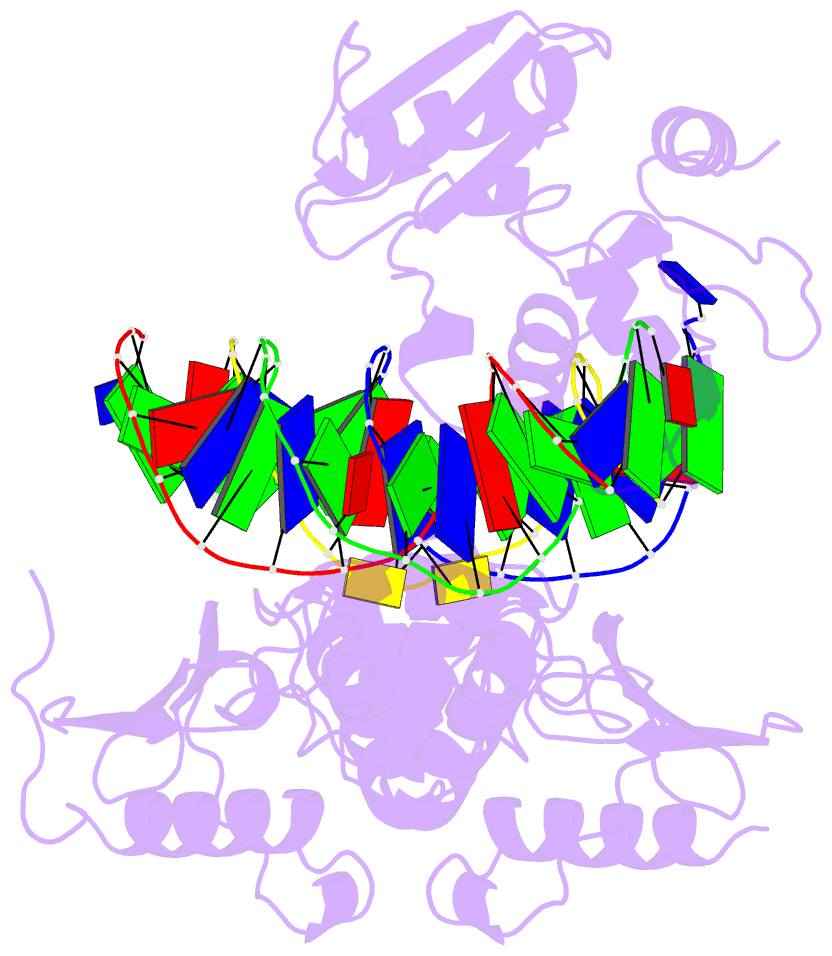
Summary information and primary citation
- PDB-id
- 1yfh; DSSR-derived features in text and JSON formats
- Class
- transferase-DNA
- Method
- X-ray (3.01 Å)
- Summary
- Wt human o6-alkylguanine-DNA alkyltransferase bound to DNA containing an alkylated cytosine
- Reference
- Duguid EM, Rice PA, He C (2005): "The structure of the human AGT protein bound to DNA and its implications for damage detection." J.Mol.Biol., 350, 657-666. doi: 10.1016/j.jmb.2005.05.028.
- Abstract
- O6-Alklyguanine-DNA alkyltransferase (AGT) is an important DNA repair protein that protects cells from mutagenesis and toxicity arising from alkylating agents. We present an X-ray crystal structure of the wild-type human protein (hAGT) bound to double-stranded DNA with a chemically modified cytosine base. The protein binds at two different sites: one at the modified base, and the other across a sticky-ended DNA junction. The protein molecule that binds the modified cytosine base flips the base and recognizes it in its active site. The one that binds ends of neighboring DNA molecules partially flips an overhanging thymine base. This base is not inserted into the active-site pocket of the protein. These two different hAGT/DNA interactions observed in the structure suggest that hAGT may not detect DNA lesions by searching for the adduct itself, but rather for weakened and/or distorted base-pairs caused by base damage in the duplex DNA. We propose that hAGT imposes a strain on the DNA duplex and searches for DNA regions where the native structure is destabilized. The structure provides implications for pyrimidine recognition, improved inhibitor design, and a possible protein/protein interaction patch on hAGT.