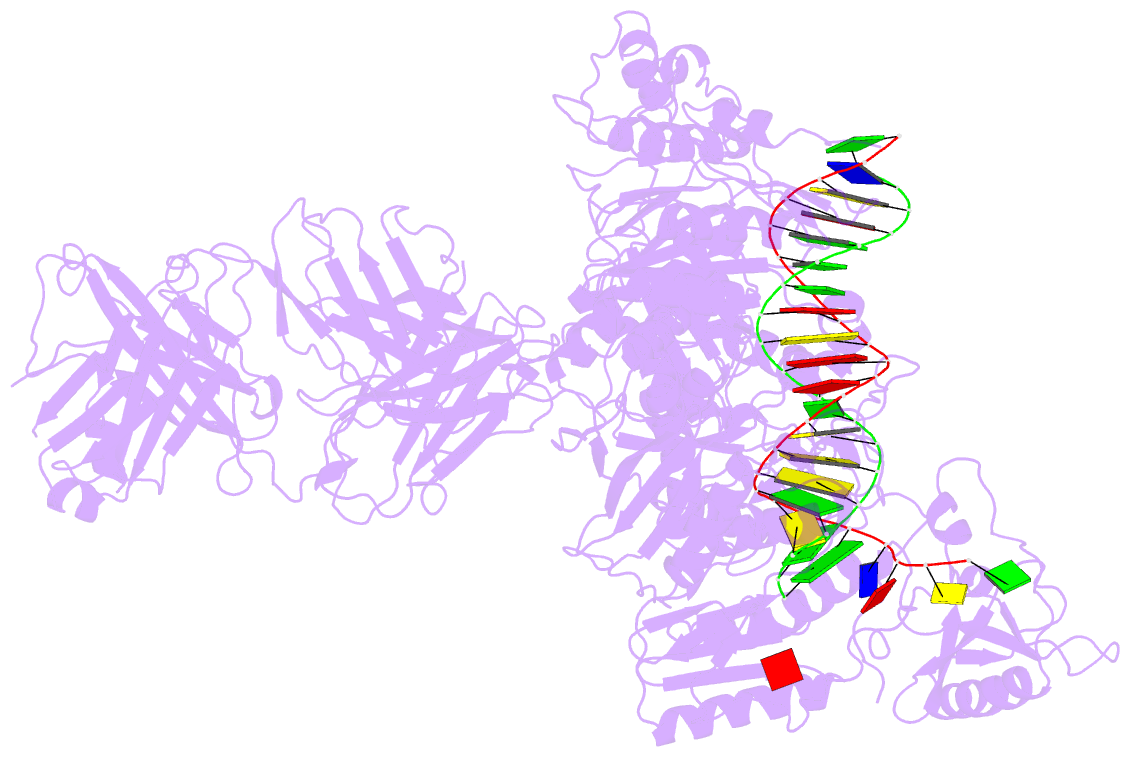
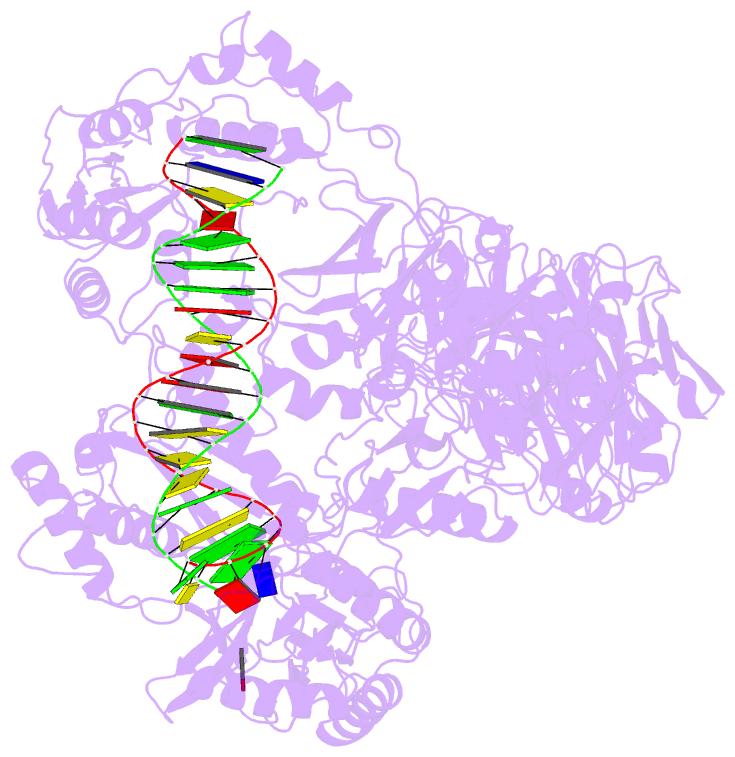
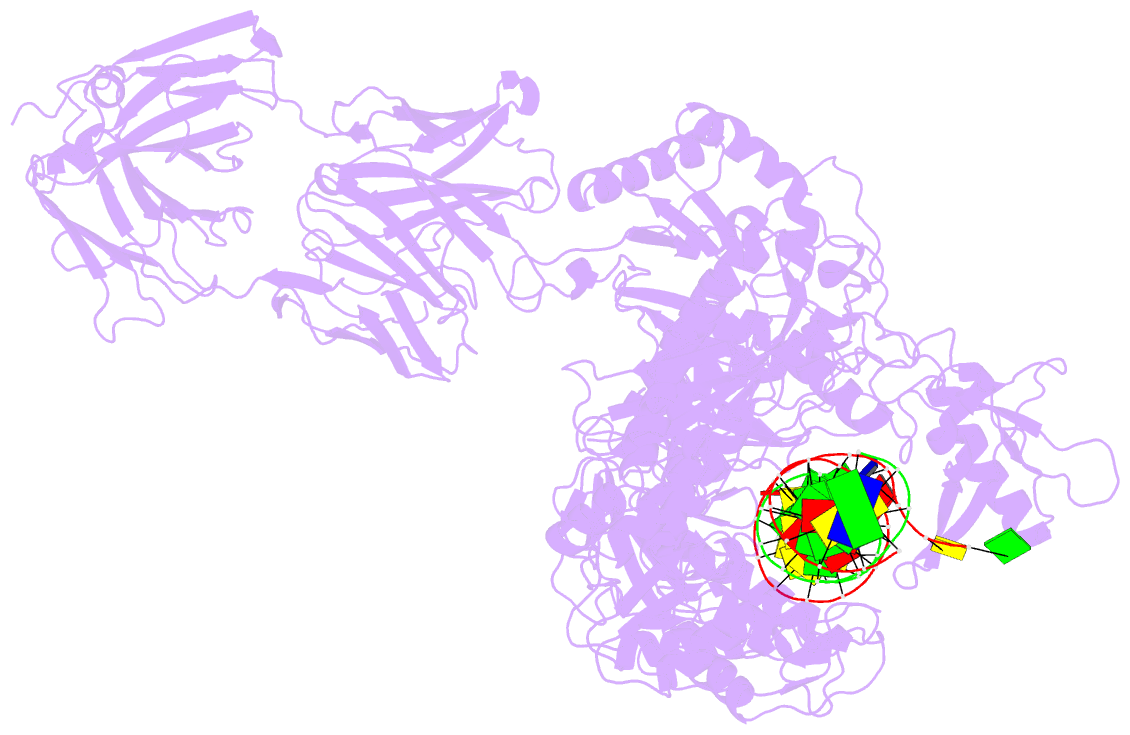
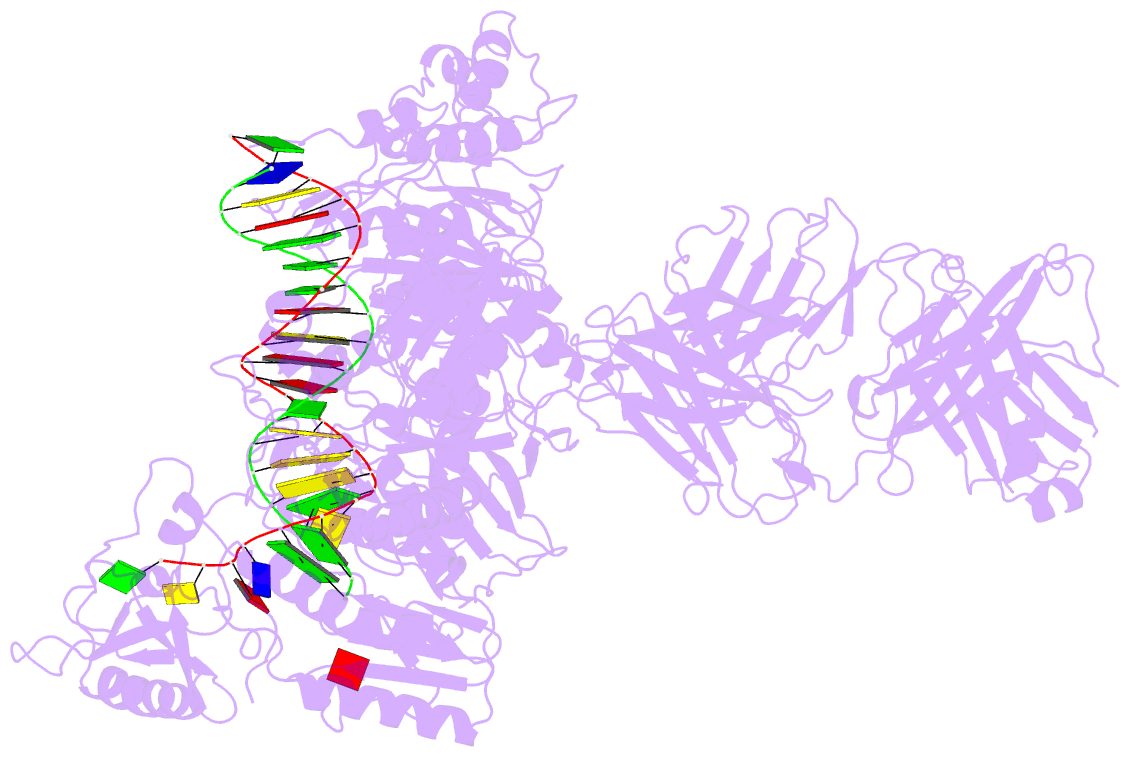
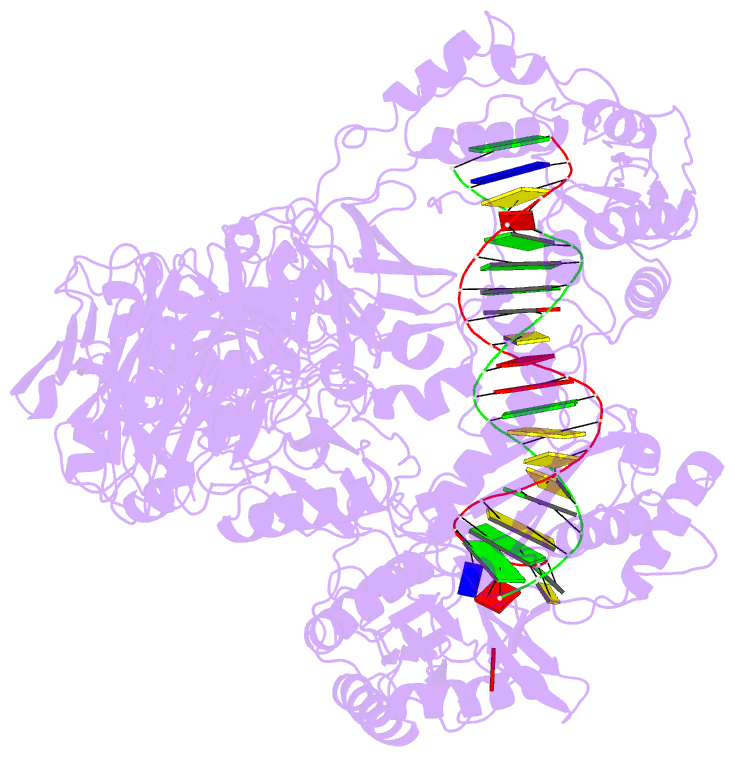
Summary information and primary citation
- PDB-id
- 1t03; DSSR-derived features in text and JSON formats
- Class
- transferase-antibody-DNA
- Method
- X-ray (3.1 Å)
- Summary
- Hiv-1 reverse transcriptase crosslinked to tenofovir terminated template-primer (complex p)
- Reference
- Tuske S, Sarafianos SG, Clark Jr AD, Ding J, Naeger LK, White KL, Miller MD, Gibbs CS, Boyer PL, Clark P, Wang G, Gaffney BL, Jones RA, Jerina DM, Hughes SH, Arnold E (2004): "Structure of HIV-1 RT-DNA complexes before and after incorporation of the anti-AIDS drug tenofovir." Nat.Struct.Mol.Biol., 11, 469-474. doi: 10.1038/nsmb760.
- Abstract
- Tenofovir, also known as PMPA, R-9-(2-(phosphonomethoxypropyl)adenine, is a nucleotide reverse transcriptase (RT) inhibitor. We have determined the crystal structures of two related complexes of HIV-1 RT with template primer and tenofovir: (i) a ternary complex at a resolution of 3.0 A of RT crosslinked to a dideoxy-terminated DNA with tenofovir-diphosphate bound as the incoming substrate; and (ii) a RT-DNA complex at a resolution of 3.1 A with tenofovir at the 3' primer terminus. The tenofovir nucleotide in the tenofovir-terminated structure seems to adopt multiple conformations. Some nucleoside reverse transcriptase inhibitors, including 3TC and AZT, have elements ('handles') that project beyond the corresponding elements on normal dNTPs (the 'substrate envelope'). HIV-1 RT resistance mechanisms to AZT and 3TC take advantage of these handles; tenofovir's structure lacks handles that could protrude through the substrate envelope to cause resistance.