Summary information and primary citation
- PDB-id
-
1kc6;
SNAP-derived features in text and
JSON formats
- Class
- hydrolase-DNA
- Method
- X-ray (2.6 Å)
- Summary
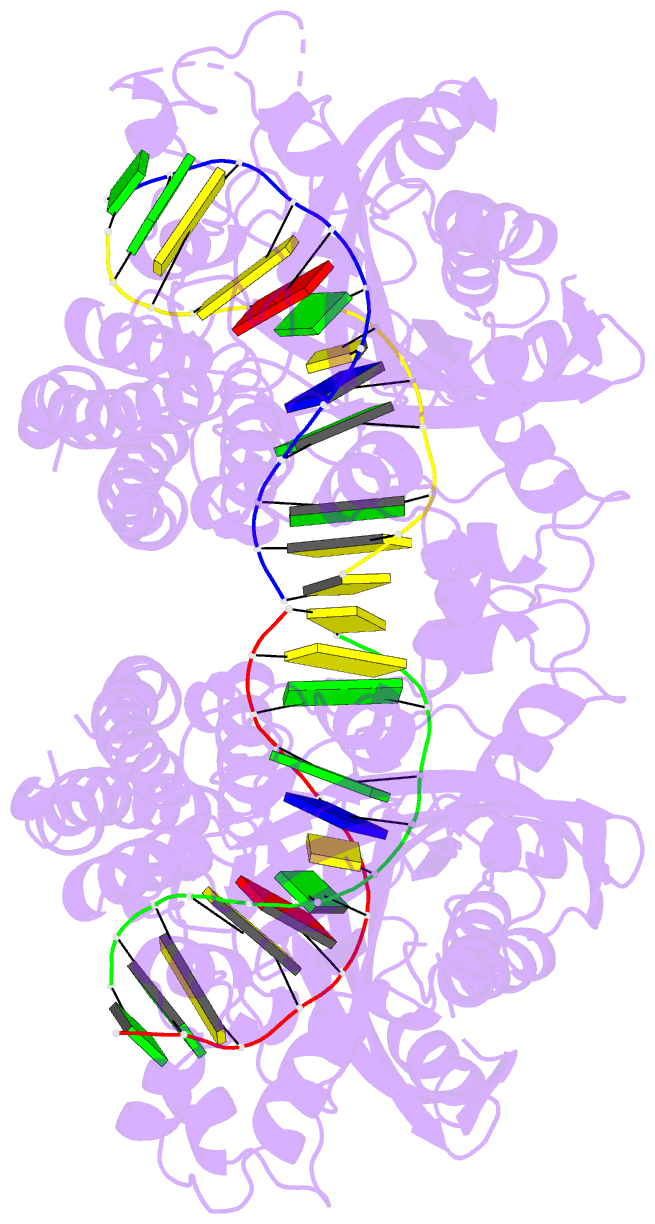
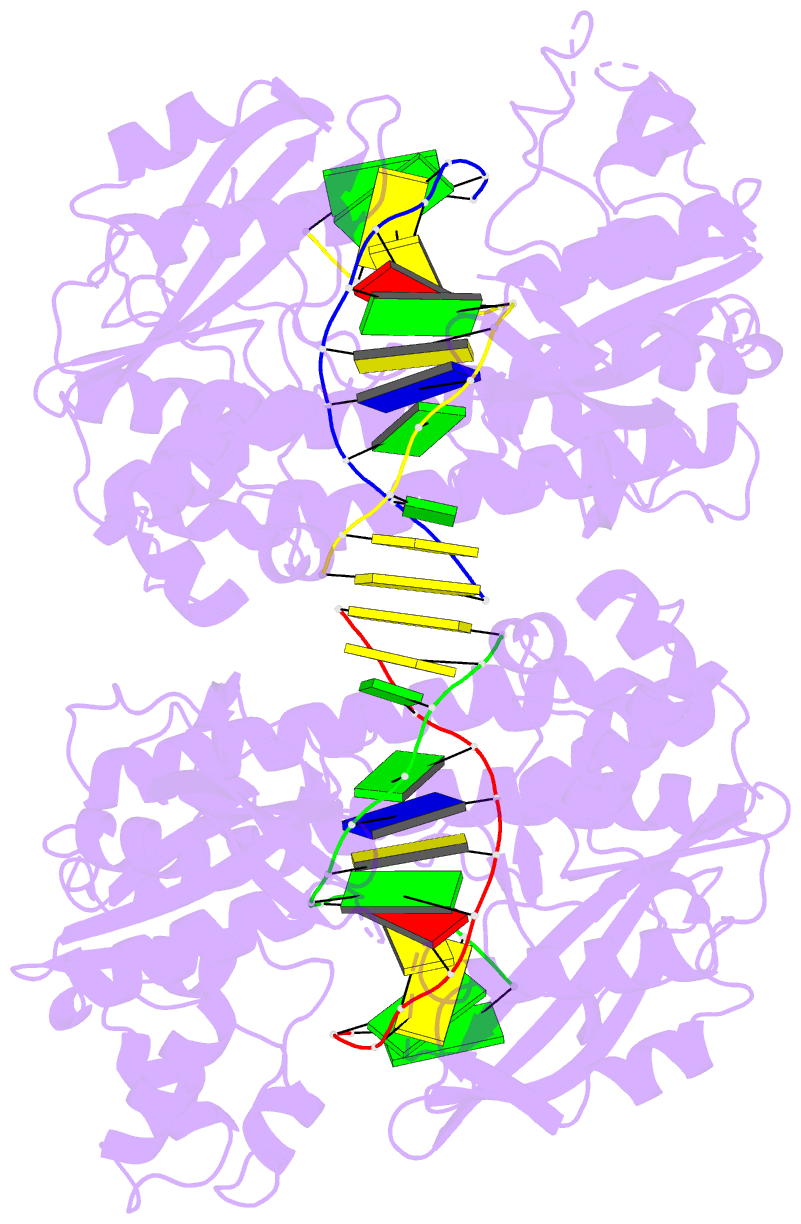
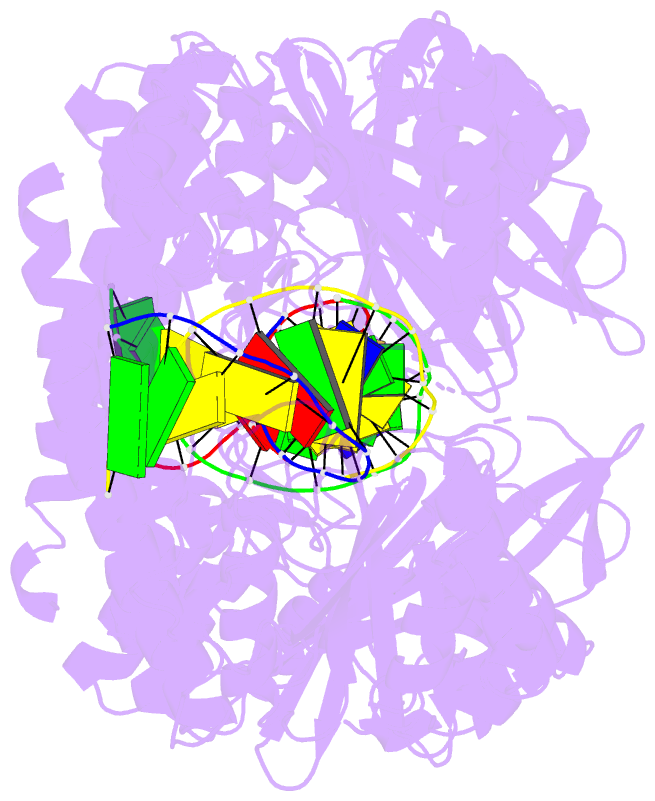
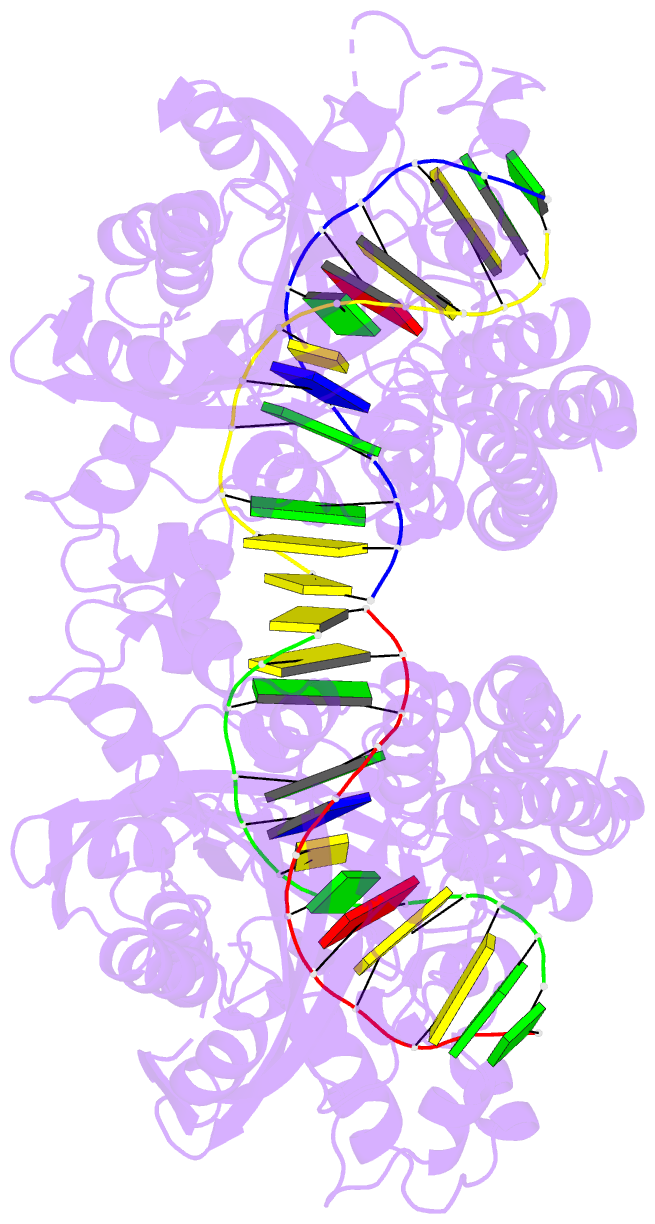
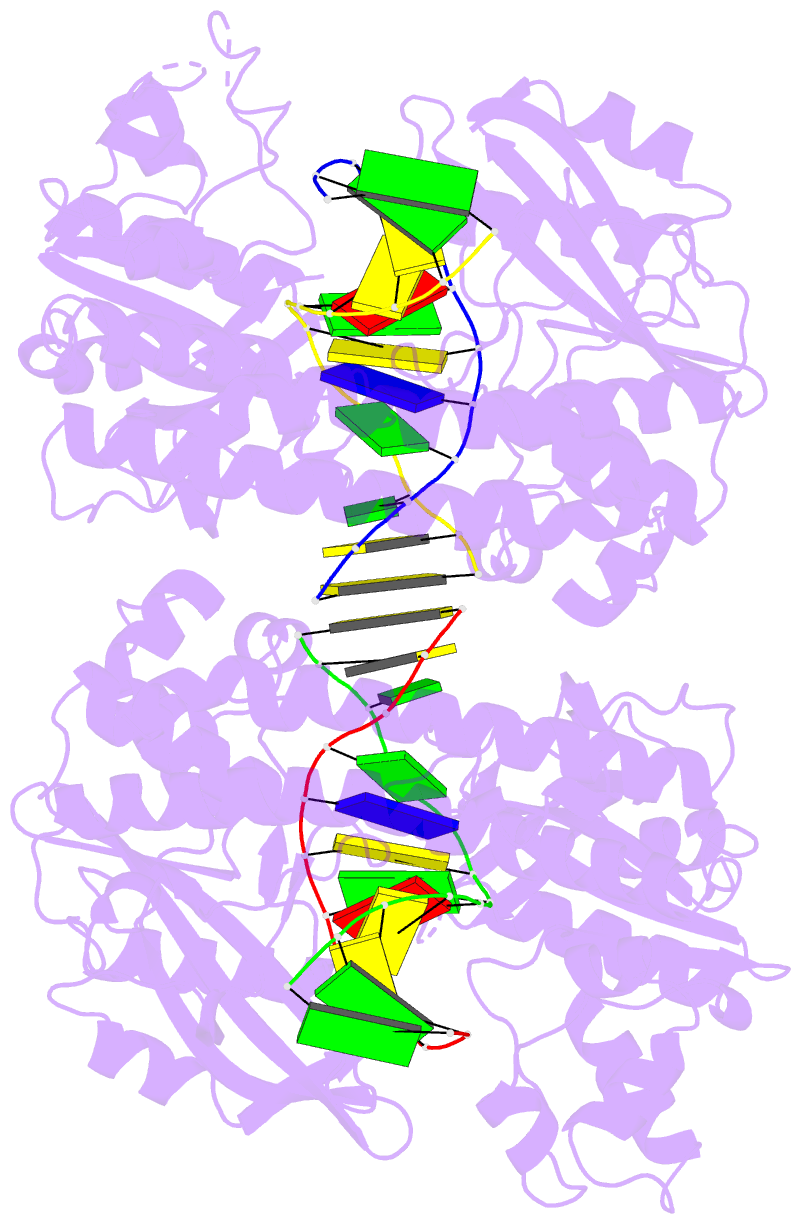
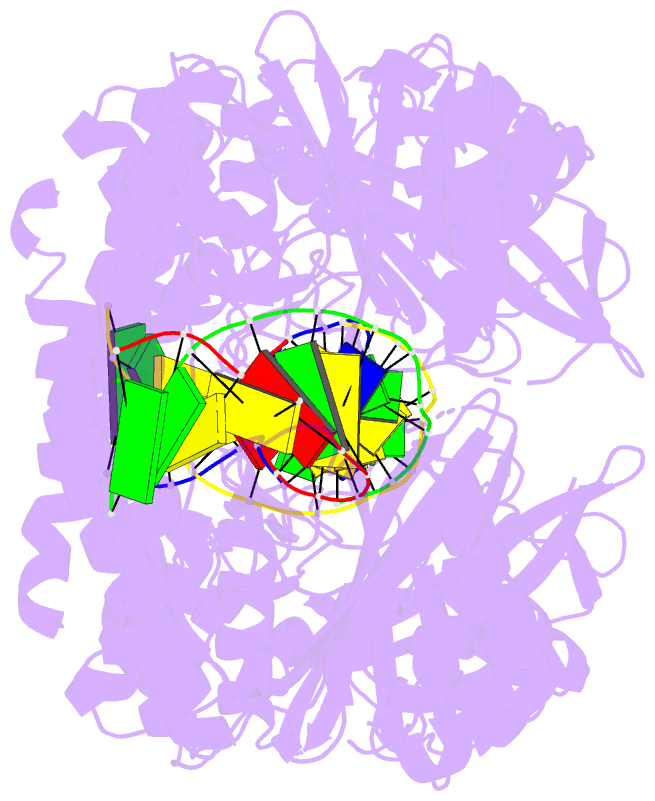
- Hincii bound to cognate DNA
- Reference
-
Horton NC, Dorner LF, Perona JJ (2002): "Sequence
selectivity and degeneracy of a restriction endonuclease
mediated by DNA intercalation."
Nat.Struct.Biol., 9, 42-47.
doi: 10.1038/nsb741.
- Abstract
- The crystal structure of the HincII restriction
endonuclease-DNA complex shows that degenerate specificity
for blunt-ended cleavage at GTPyPuAC sequences arises from
indirect readout of conformational preferences at the
center pyrimidine-purine step. Protein-induced distortion
of the DNA is accomplished by intercalation of glutamine
side chains into the major groove on either side of the
recognition site, generating bending by either tilt or roll
at three distinct loci. The intercalated side chains
propagate a concerted shift of all six target-site base
pairs toward the minor groove, producing an unusual
cross-strand purine stacking at the center
pyrimidine-purine step. Comparison of the HincII and EcoRV
cocrystal structures suggests that sequence-dependent
differences in base-stacking free energies are a crucial
underlying factor mediating protein recognition by indirect
readout.