Summary information and primary citation
- PDB-id
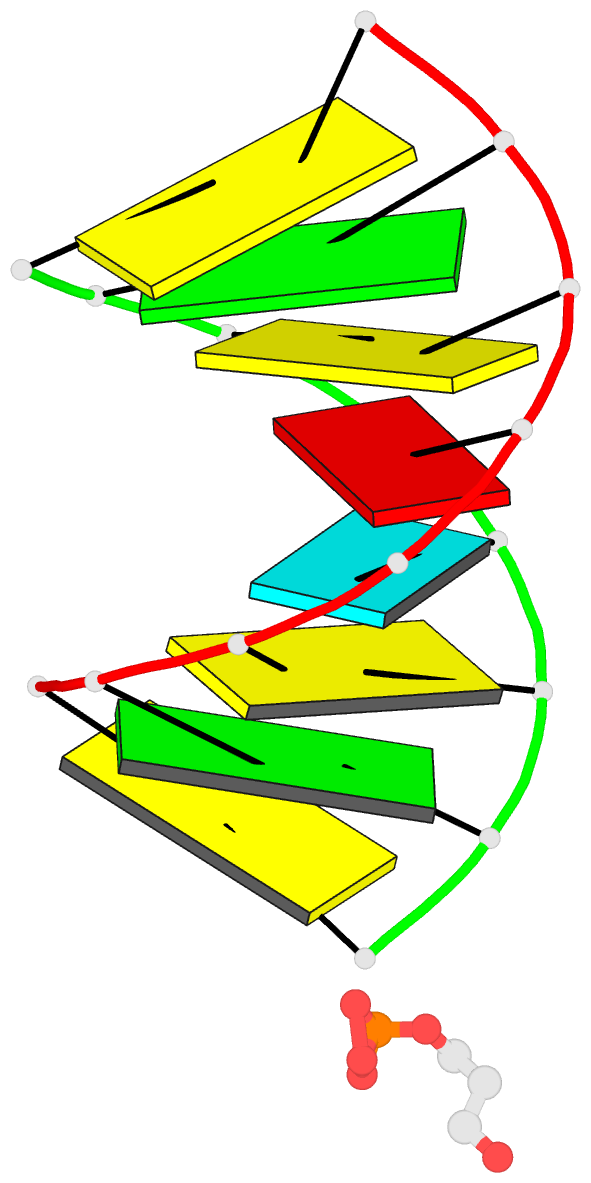
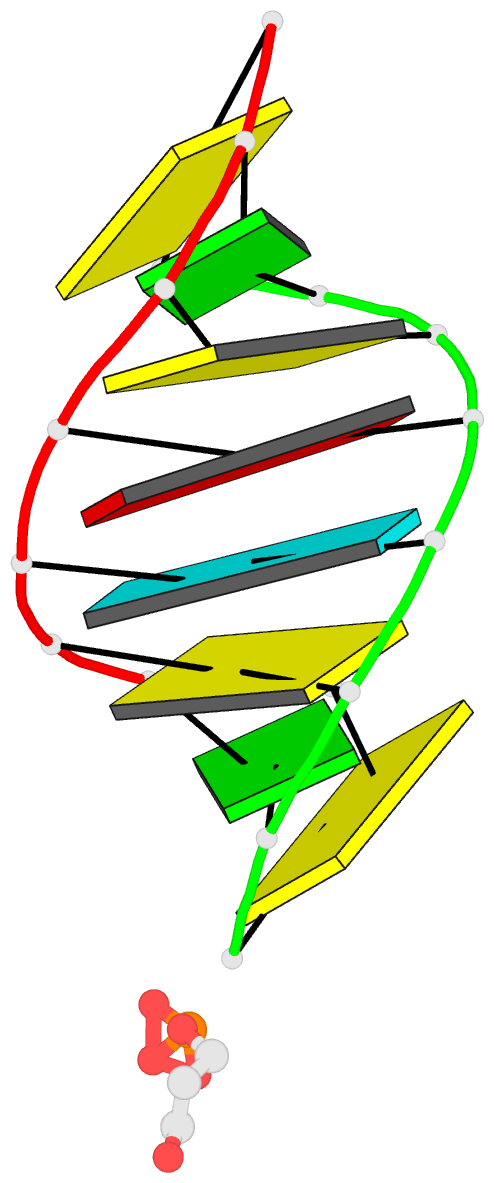
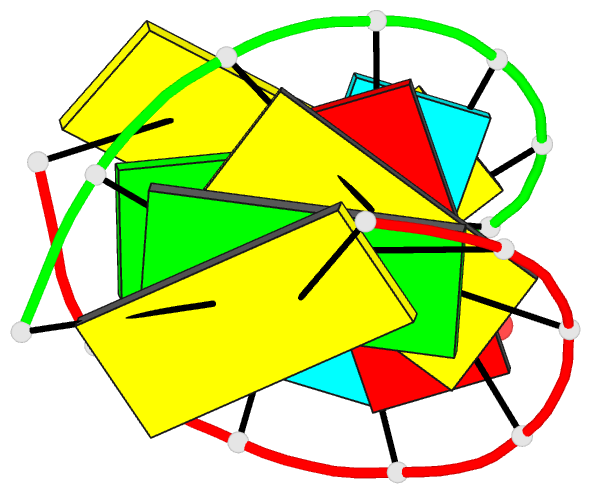
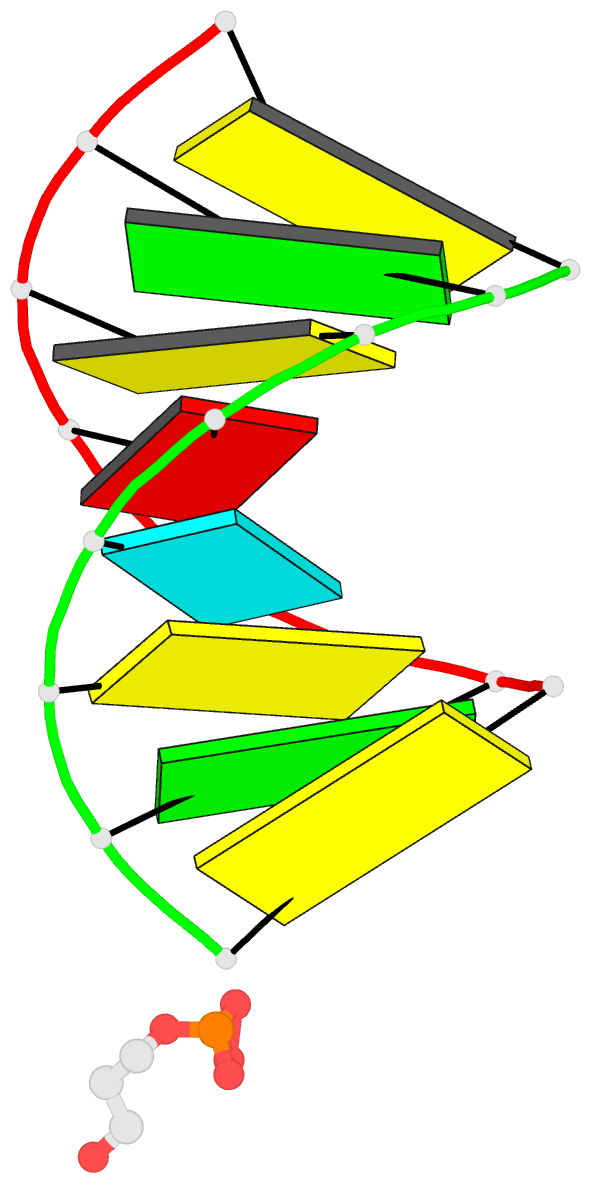
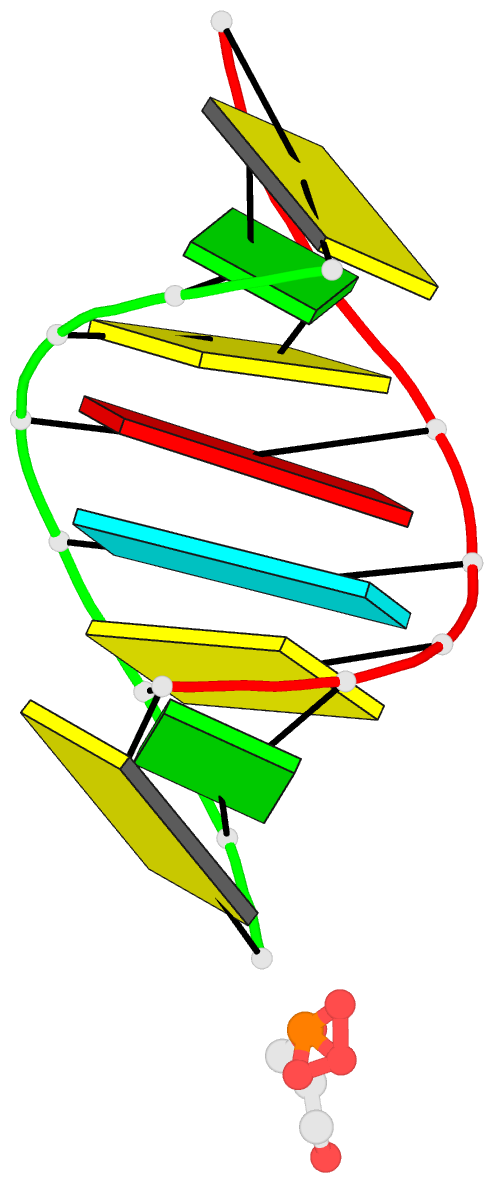
- 1ejz; DSSR-derived features in text and JSON formats
- Class
- RNA
- Method
- NMR
- Summary
- Solution structure of a hna-RNA hybrid
- Reference
- Lescrinier E, Esnouf R, Schraml J, Busson R, Heus H, Hilbers C, Herdewijn P (2000): "Solution structure of a HNA-RNA hybrid." Chem.Biol., 7, 719-731. doi: 10.1016/S1074-5521(00)00017-X.
- Abstract
- Background: Synthetic nucleic acid analogues with a conformationally restricted sugar-phosphate backbone are widely used in antisense strategies for biomedical and biochemical applications. The modified backbone protects the oligonucleotides against degradation within the living cell, which allows them to form stable duplexes with sequences in target mRNAs with the aim of arresting their translation. The biologically most active antisense oligonucleotides also trigger cleavage of the target RNA through activation of endogenous RNase H. Systematic studies of synthetic oligonucleotides have also been conducted to delineate the origin of the chirality of DNA and RNA that are both composed of D-nucleosides.
Results: Hexitol nucleic acids (HNA) are the first example of oligonucleotides with a six-membered carbohydrate moiety that can bind strongly and selectively to complementary RNA oligomers. We present the first high resolution nuclear magnetic resonance structure of a HNA oligomer bound to a complementary RNA strand. The HNA-RNA complex forms an anti-parallel heteroduplex and adopts a helical conformation that belongs to the A-type family. Possibly, due to the rigidity of the rigid chair conformation of the six-membered ring both the HNA and RNA strand in the duplex are well defined. The observed absence of end-fraying effects also indicate a reduced conformational flexibility of the HNA-RNA duplex compared to canonical dsRNA or an RNA-DNA duplex.
Conclusions: The P-P distance across the minor groove, which is close to A-form, and the rigid conformation of the HNA-RNA complex, explain its resistance towards degradation by Rnase H. The A-form character of the HNA-RNA duplex and the reduced flexibility of the HNA strand is possibly responsible for the stereoselectivity of HNA templates in non-enzymatic replication of oligonucleotides, supporting the theory that nucleosides with six-membered rings could have existed at some stage in molecular evolution.