Summary information and primary citation
- PDB-id
- 1dl4; DSSR-derived features in text and JSON formats
- Class
- DNA
- Method
- NMR
- Summary
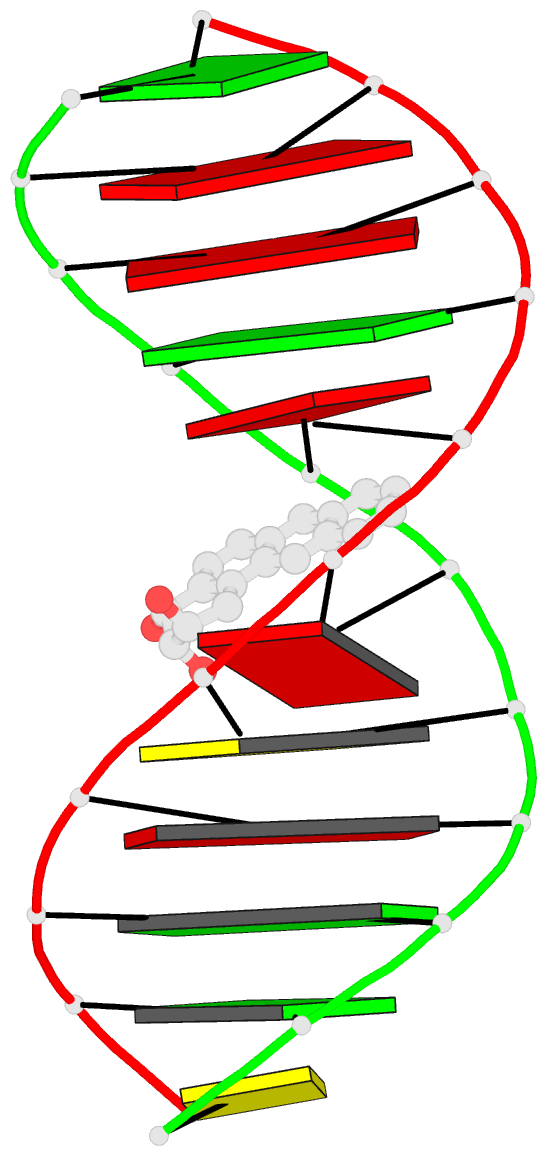
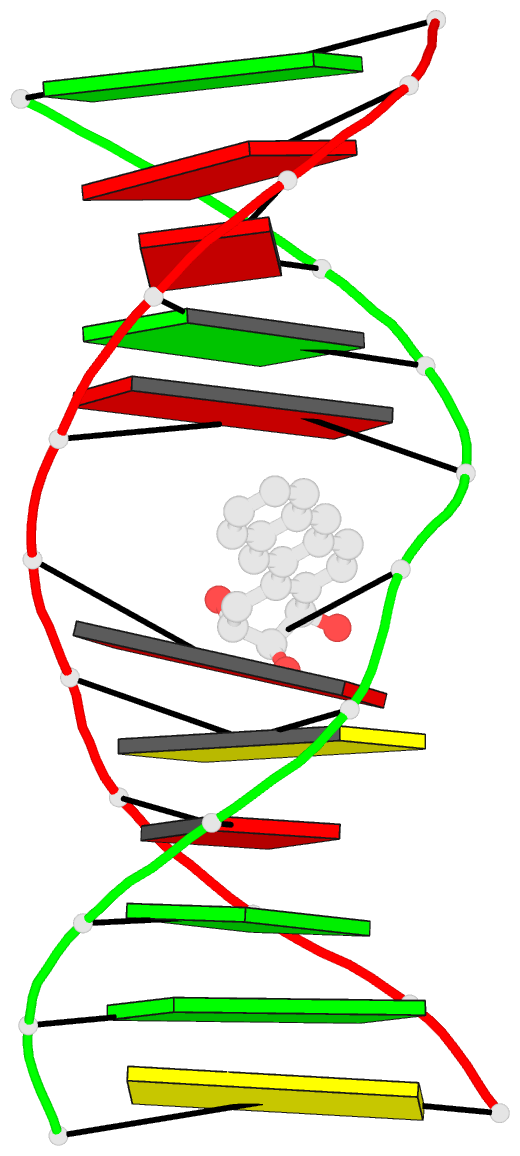
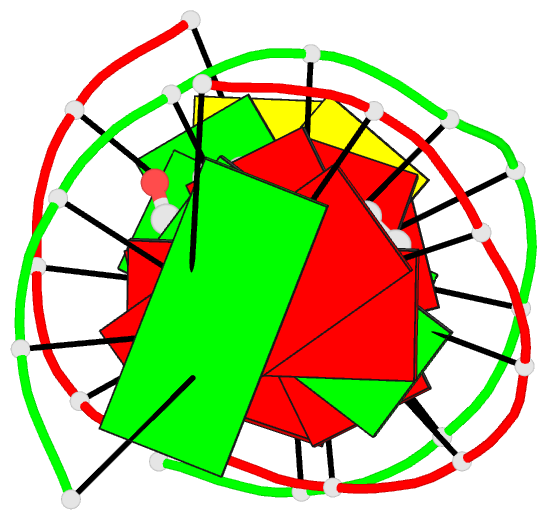
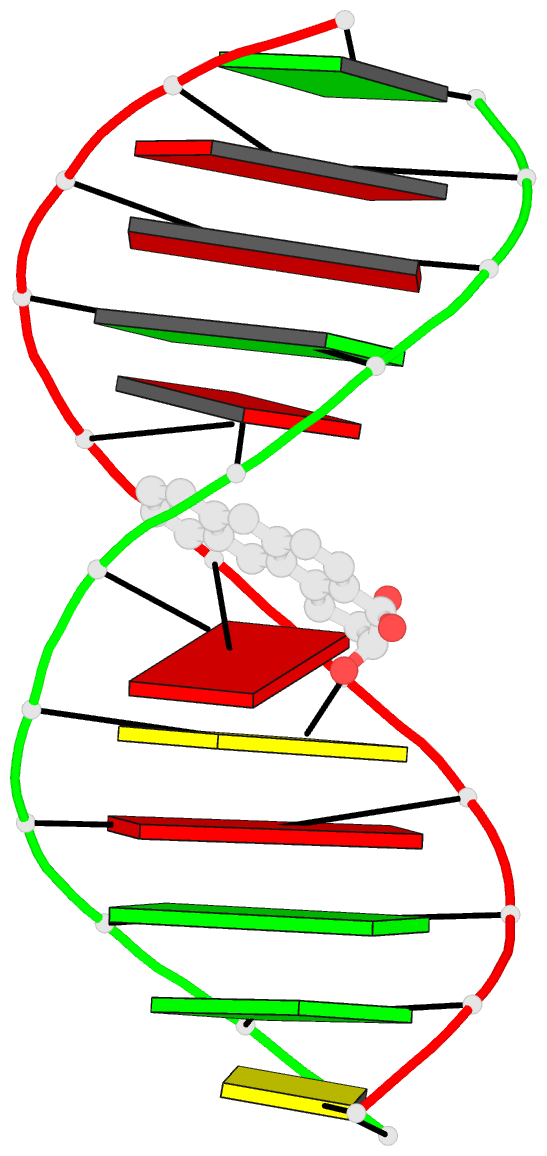
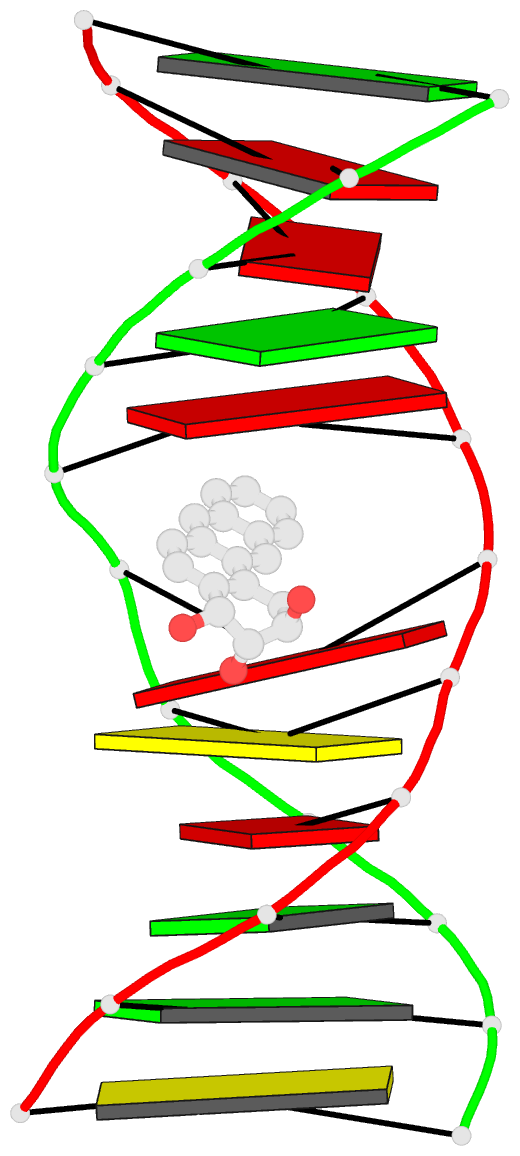
- The solution structure of a bay-region 1s-benz[a]anthracene oxide adduct at the n6 position of adenine of an oligodeoxynucleotide containing the human n-ras codon 61 sequence
- Reference
- Li Z, Kim HY, Tamura PJ, Harris CM, Harris TM, Stone MP (1999): "Intercalation of the (1S,2R,3S,4R)-N6-[1-(1,2,3,4-tetrahydro-2,3, 4-trihydroxybenz[a]anthracenyl)]-2'-deoxyadenosyl adduct in an oligodeoxynucleotide containing the human N-ras codon 61 sequence." Biochemistry, 38, 16045-16057. doi: 10.1021/bi9903650.
- Abstract
- The (1S,2R,3S,4R)-N(6)-[1-(1,2,3,4-tetrahydro-2,3, 4-trihydroxybenz[a]anthracenyl)]-2'-deoxyadenosyl adduct at X6 of 5'-d(CGGACXAGAAG)-3'.5'-d(CTTCTTGTCCG)-3', incorporating codons 60, 61 (underlined), and 62 of the human N-ras protooncogene, results from trans opening of (1R,2S,3S,4R)-1,2-epoxy-1,2,3, 4-tetrahydrobenz[a]anthracenyl-3,4-diol by the exocyclic N6 of adenine. Two conformations of this adduct exist, in slow exchange on the NMR time scale. A structure for the major conformation, which represents approximately 80% of the population, is presented. In this conformation, an anti glycosidic torsion angle is observed for all nucleotides, including S,R,S,RA6. The refined structure is a right-handed duplex, with the benz[a]anthracene moiety intercalated on the 3'-face of the modified base pair, from the major groove. It is located between S,R,S,RA6.T17 and A7.T16. Intercalation is on the opposite face of the modified S,R,S,RA6.T17 base pair as compared to the (1R,2S,3R,4S)-N6-[1-(1,2,3,4-tetrahydro-2, 3,4-trihydroxybenz[a]anthracenyl)]-2'-deoxyadenosyl adduct, which intercalated 5' to the modified R,S,R,SA6.T17 base pair [Li, Z. , Mao, H., Kim, H.-Y., Tamura, P. J., Harris, C. M., Harris, T. M., and Stone, M. P. (1999) Biochemistry 38, 2969-2981]. The spectroscopic data do not allow refinement of the minor conformation, but suggest that the adenyl moiety in the modified nucleoti111S,R, S,RA6 adopts a syn glycosidic torsion angle. Thus, the minor conformation may create greater distortion of the DNA duplex. The results are discussed in the context of site-specific mutagenesis studies which reveal that the S,R,S,RA6 lesion is less mutagenic than the R,S,R,SA6 lesion.