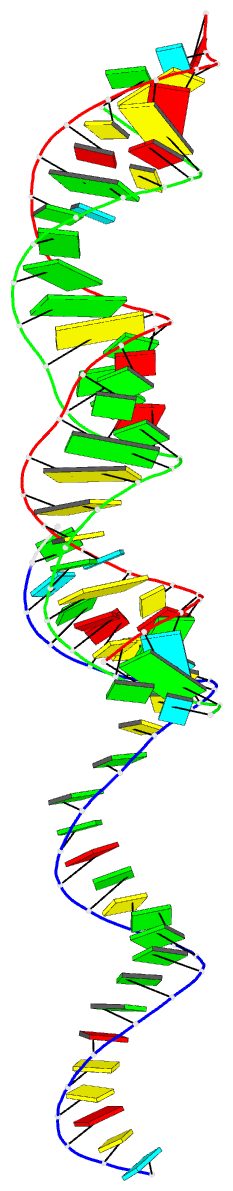
Summary information and primary citation
- PDB-id
- 1d4r; DSSR-derived features in text and JSON formats
- Class
- RNA
- Method
- X-ray (2.0 Å)
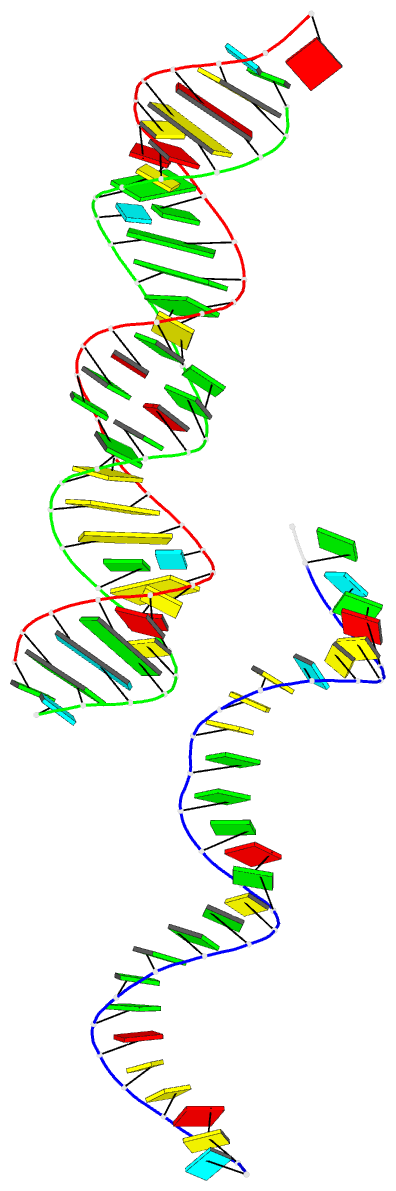
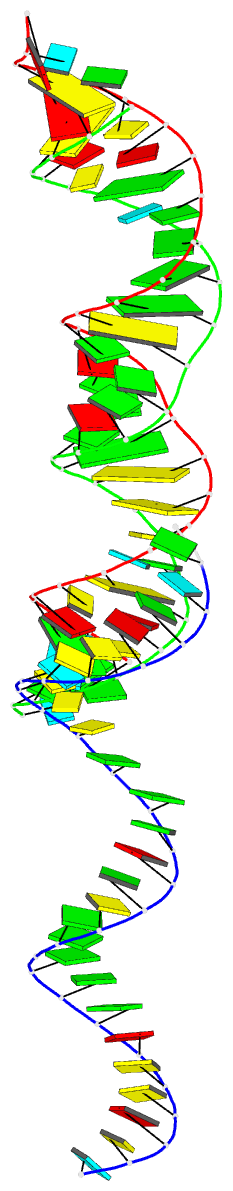
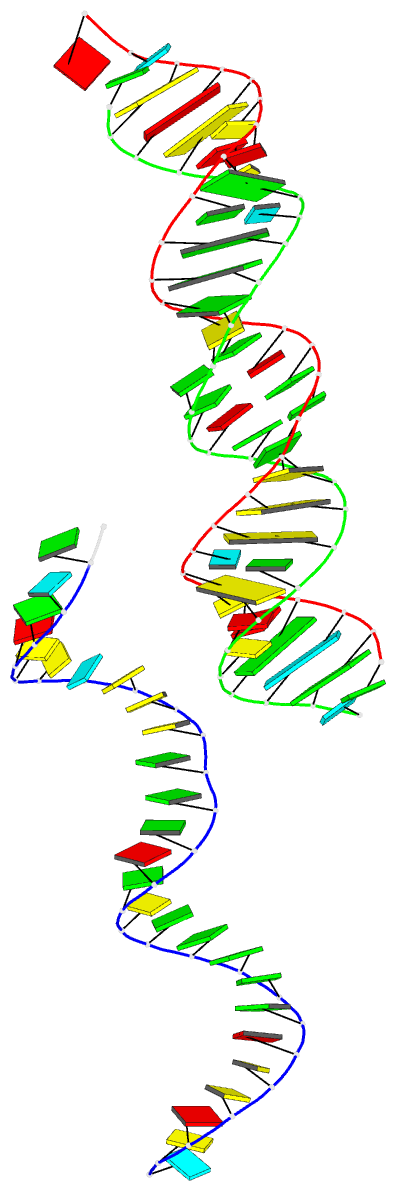
- Summary
- 29-mer fragment of human srp RNA helix 6
- Reference
- Wild K, Weichenrieder O, Leonard GA, Cusack S (1999): "The 2 A structure of helix 6 of the human signal recognition particle RNA." Structure Fold.Des., 7, 1345-1352. doi: 10.1016/S0969-2126(00)80024-6.
- Abstract
- Background: The mammalian signal recognition particle (SRP) is an essential cytoplasmic ribonucleoprotein complex involved in targeting signal-peptide-containing proteins to the endoplasmic reticulum. Assembly of the SRP requires protein SRP19 to bind first to helix 6 of the SRP RNA before the signal-peptide-recognizing protein, SRP54, can bind to helix 8 of the RNA. Helix 6 is closed by a GGAG tetraloop, which has been shown to form part of the SRP19-binding site.
Results: The high-resolution (2.0 A) structure of a fragment of human SRP RNA comprising 29 nucleotides of helix 6 has been determined using the multiple anomalous dispersion (MAD) method and bromine-labelled RNA. In the crystal the molecule forms 28-mer duplexes rather than the native monomeric hairpin structure, although two chemically equivalent 11 base pair stretches of the duplex represent the presumed native structure. The duplex has highly distorted A-RNA geometry caused by the occurrence of several non-Watson-Crick base pairs. These include a 5'-GGAG-3'/3'-GAGG-5' purine bulge (which replaces the tetraloop) and a 5'-AC-3'/3'-CA-5' tandem mismatch that, depending on the protonation state of the adenine bases, adopts a different conformation in the two native-like parts of the structure. The structure also shows the 2'3'-cyclic phosphate reaction product of the hammerhead ribozyme cleavage reaction.
Conclusions: The 29-mer RNA is the first RNA structure of the human SRP and provides some insight into the binding mode of SRP19. The observed strong irregularities of the RNA helix make the major groove wide enough and flat enough to possibly accommodate an alpha helix of SRP19. The variety of non-canonical base pairs observed enlarges the limited repertoire of irregular RNA folds known to date and the observed conformation of the 2'3'-cyclic phosphate containing Ade29 is consistent with the current understanding of the hammerhead ribozyme reaction mechanism.