Summary information and primary citation
- PDB-id
- 1axl; DSSR-derived features in text and JSON formats
- Class
- DNA
- Method
- NMR
- Summary
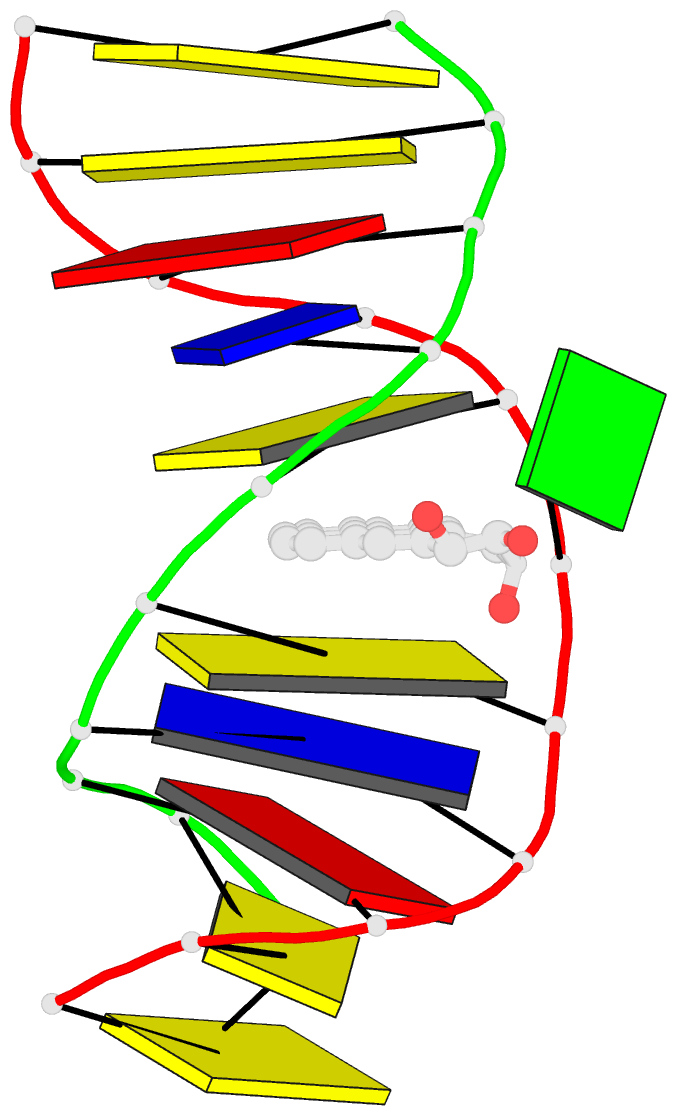
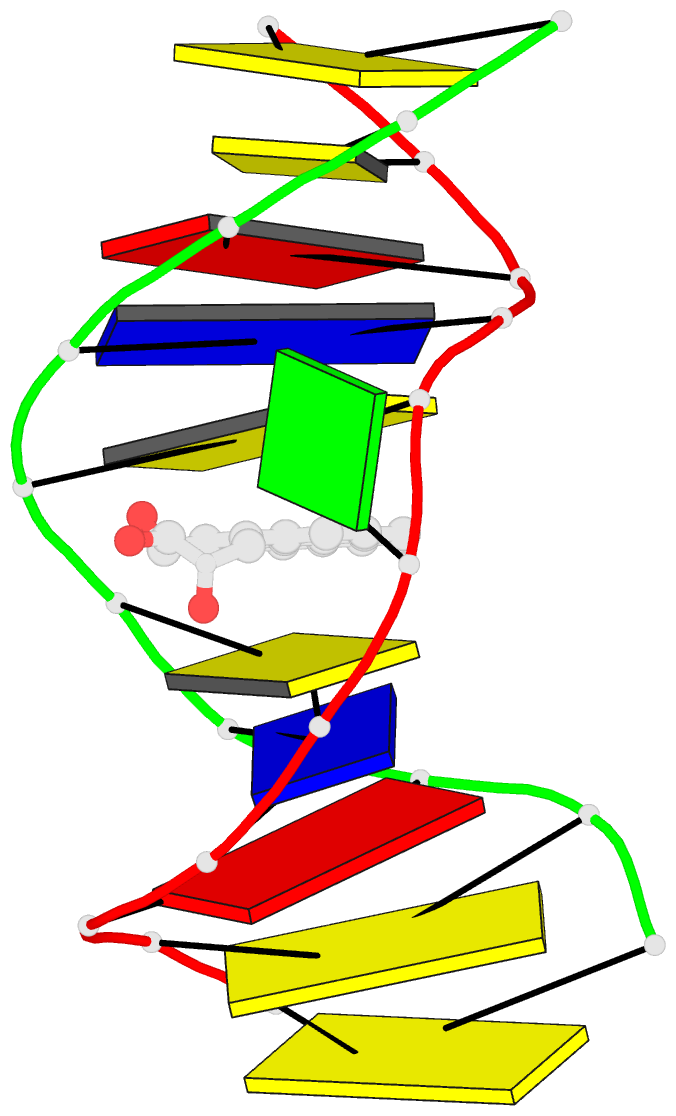
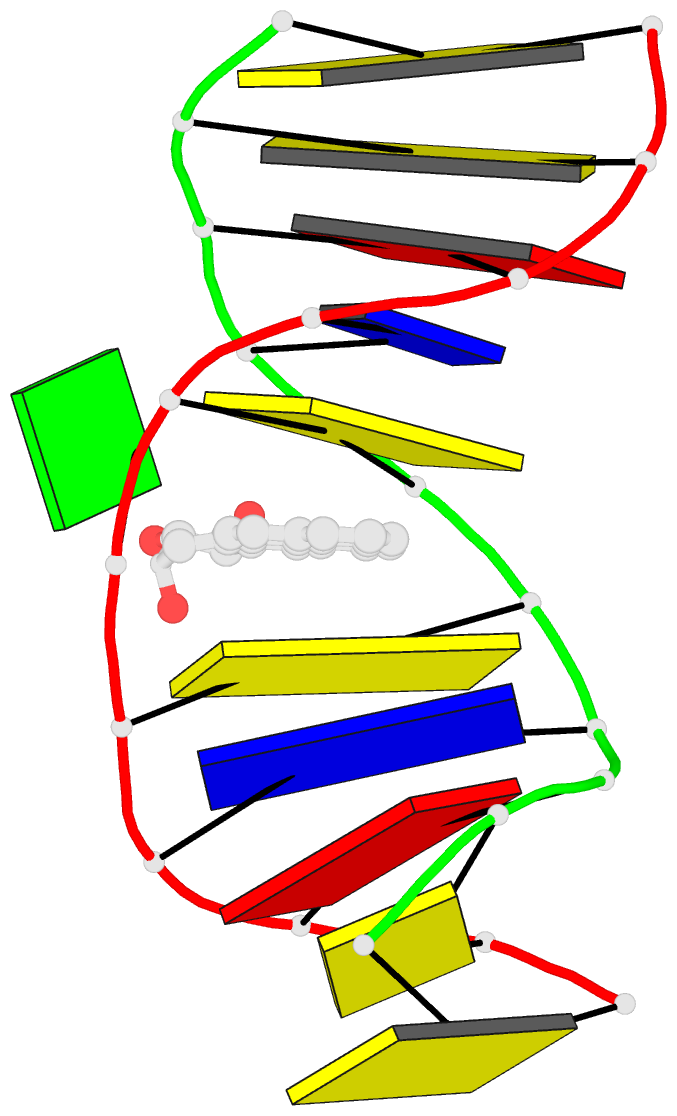
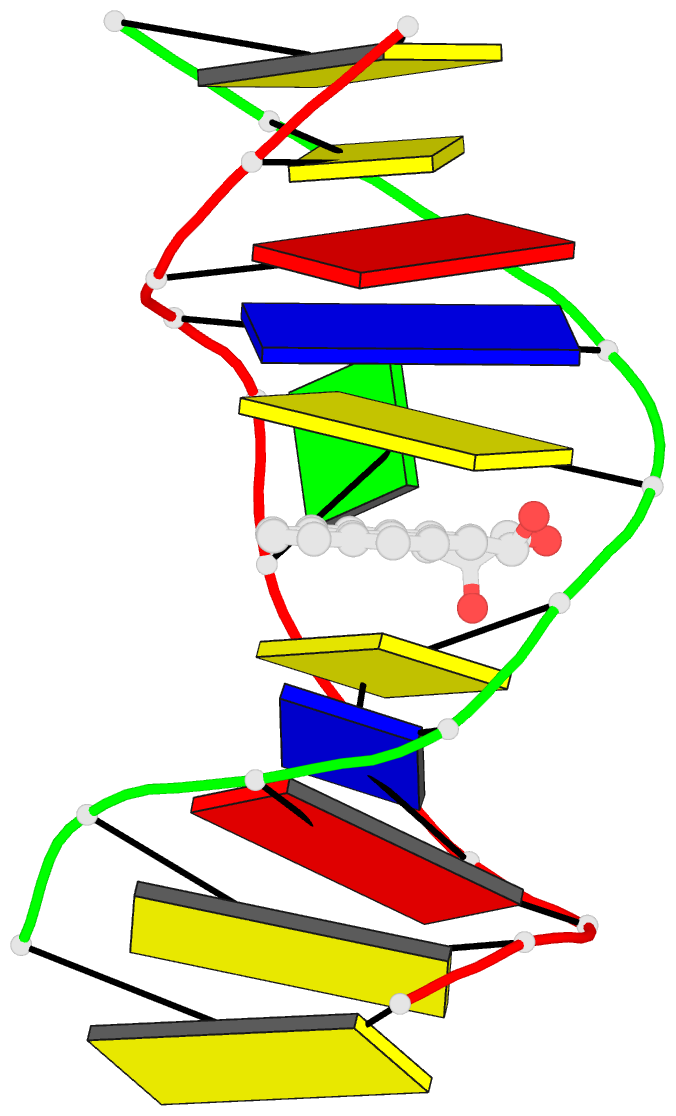
- Solution conformation of the (-)-trans-anti-[bp]dg adduct opposite a deletion site in DNA duplex d(ccatc-[bp]g-ctacc)d(ggtag--gatgg), NMR, 6 structures
- Reference
- Feng B, Gorin A, Kolbanovskiy A, Hingerty BE, Geacintov NE, Broyde S, Patel DJ (1997): "Solution conformation of the (-)-trans-anti-[BP]dG adduct opposite a deletion site in a DNA duplex: intercalation of the covalently attached benzo[a]pyrene into the helix with base displacement of the modified deoxyguanosine into the minor groove." Biochemistry, 36, 13780-13790. doi: 10.1021/bi970070r.
- Abstract
- A combined NMR-computational approach was employed to determine the solution structure of the (-)-trans-anti-[BP]dG adduct positioned opposite a -1 deletion site in the d(C1-C2-A3-T4-C5- [BP]G6-C7-T8-A9-C10-C11).d(G12-G13-T14-A15-G1 6-G17-A18-T19-G20-G21) sequence context. The (-)-trans-anti-[BP]dG moiety is derived from the binding of the (-)-anti-benzo[a]pyrene diol epoxide [(-)-anti-BPDE] to N2 of dG6 and has a 10R absolute configuration at the [BP]dG linkage site. The exchangeable and non-exchangeable protons of the benzo[a]pyrenyl moiety and the nucleic acid were assigned following analysis of two-dimensional NMR data sets in H2O and D2O solution. The solution conformation has been determined by incorporating intramolecular and intermolecular proton-proton distances defined by lower and upper bounds deduced from NOESY spectra as restraints in molecular mechanics computations in torsion angle space followed by restrained molecular dynamics calculations based on a NOE distance and intensity refinement protocol. Our structural studies establish that the aromatic BP ring system intercalates into the helix opposite the deletion site, while the modified deoxyguanosine residue is displaced into the minor groove with its face parallel to the helix axis. The intercalation site is wedge-shaped and the BP aromatic ring system stacks over intact flanking Watson-Crick dG.dC base pairs. The modified deoxyguanosine stacks over the minor groove face of the sugar ring of the 5'-flanking dC5 residue. The BP moiety is positioned with the benzylic ring oriented toward the minor groove and the distal pyrenyl aromatic ring directed toward the major groove. This conformation strikingly contrasts with the corresponding structure in the full duplex with the same 10R (-)-trans-anti-[BP]dG lesion positioned opposite a complementary dC residue [de los Santos et al. (1992) Biochemistry 31, 5245-5252); in this case the aromatic BP ring system is located in the minor groove, and there is no disruption of the [BP]dG.dC Watson-Crick base pairing alignment. The intercalation-base displacement features of the 10R (-)-trans-anti-[BP]dG adduct opposite a deletion site have features in common to those of the 10S (+)-trans-anti-[BP]dG adduct opposite a deletion site previously reported by Cosman et al. [(1994)(Biochemistry 33, 11507-11517], except that there is a nearly 180 degrees rotation of the BP residue about the axis of the helix at the base-displaced intercalation site and the modified deoxyguanosine is positioned in the opposite groove. In the 10S adduct, the benzylic ring is in the major groove and the aromatic ring systems point toward the minor groove. This work extends the theme of opposite orientations of adducts derived from chiral pairs of (+)- and (-)-anti-BPDE enantiomers; both 10S and 10R adducts can be positioned with opposite orientations either in the minor groove or at base displaced intercalation sites, depending on the presence or absence of the partner dC base in the complementary strand.