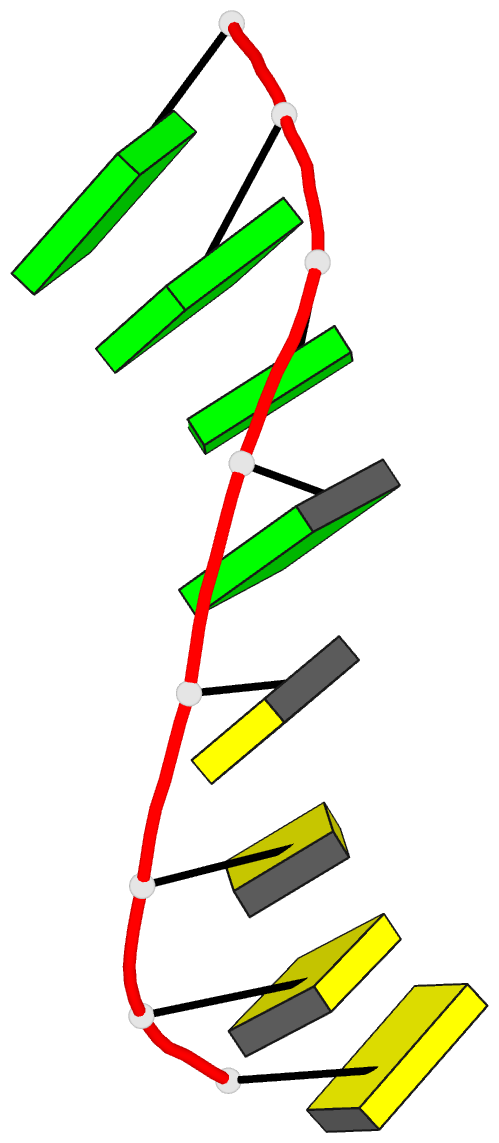
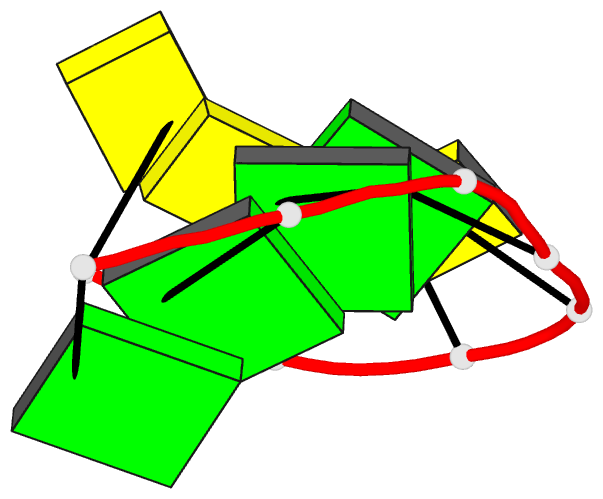
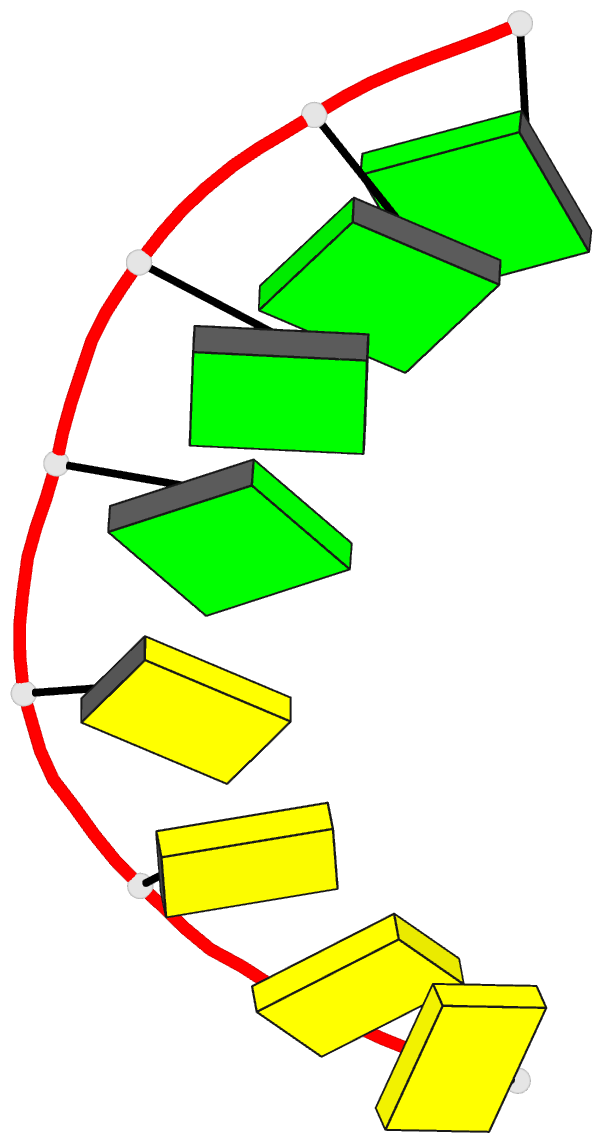
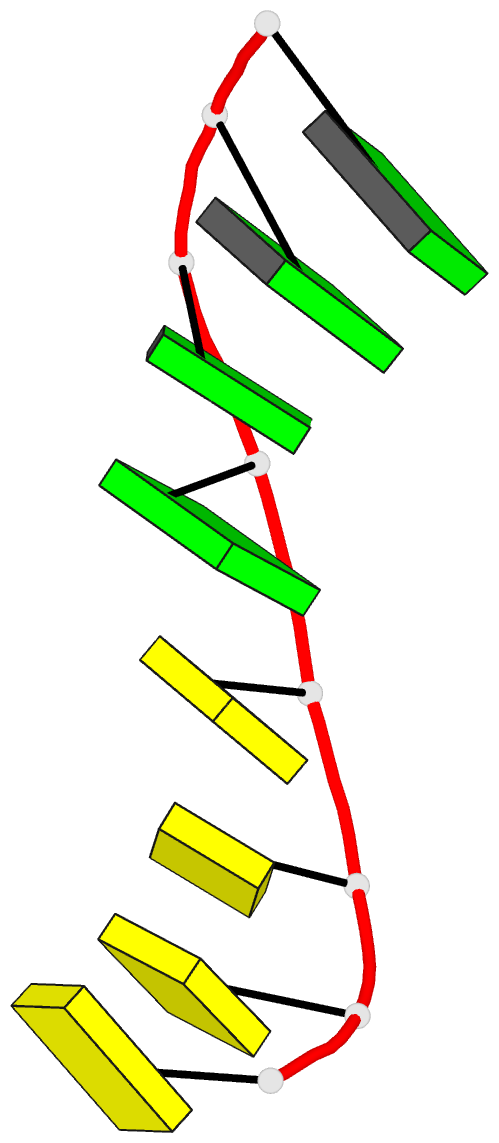
Summary information and primary citation
- PDB-id
-
187d;
SNAP-derived features in text and
JSON formats
- Class
- DNA
- Method
- X-ray (2.25 Å)
- Summary
- Hydration patterns and intermolecular interactions in
a-DNA crystal structures. implications for DNA
recognition
- Reference
-
Eisenstein M, Shakked Z (1995): "Hydration
patterns and intermolecular interactions in A-DNA crystal
structures. Implications for DNA recognition."
J.Mol.Biol., 248, 662-678. doi:
10.1006/jmbi.1995.0250.
- Abstract
- Crystallographic studies of DNA fragments of the A and
B conformations have shown that the structure and hydration
of the DNA double helix depend both on the base sequence
and the environment. Detailed analyses of solvent
organization in DNA crystals and its role in intermolecular
interactions have been reported mainly for B-DNA
structures. We have determined the crystal structures of
several isomorphous A-DNA octamers at resolutions from 1.8
to 2.5 A and refined them by the same procedure.
Comparative analysis of five independently refined
structures in terms of hydration and intermolecular
interactions has been performed leading to the following
findings. The A-DNA major groove is extensively hydrated
and together with the hydration shells of the
sugar-phosphate backbone can form an ordered network of
fused polygons. The water structure of the phosphate
backbone is less conserved than that of the grooves.
Characteristic hydration patterns are associated with
specific base sequences. The A-DNA minor groove provides
sites for intermolecular contacts through hydrophobic and
polar interactions. Well-ordered water molecules mediate
interduplex interactions that involve either the grooves or
the backbone, or both. The direct and water-mediated
intermolecular interactions observed in the A-DNA crystal
structures are relevant to various recognition motifs
between DNA and other molecules. In particular,
intermolecular interactions at the DNA minor groove are
analogous to those observed in the recently reported
crystal structures of complexes between the TATA-binding
protein and the TATA-box.