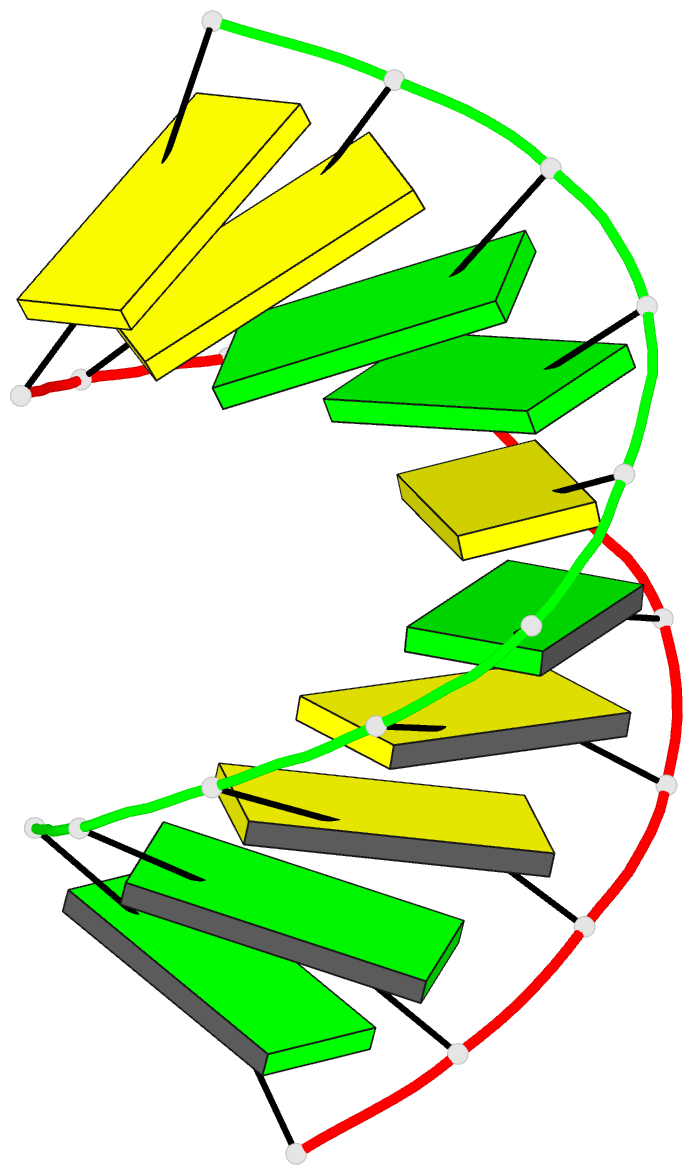
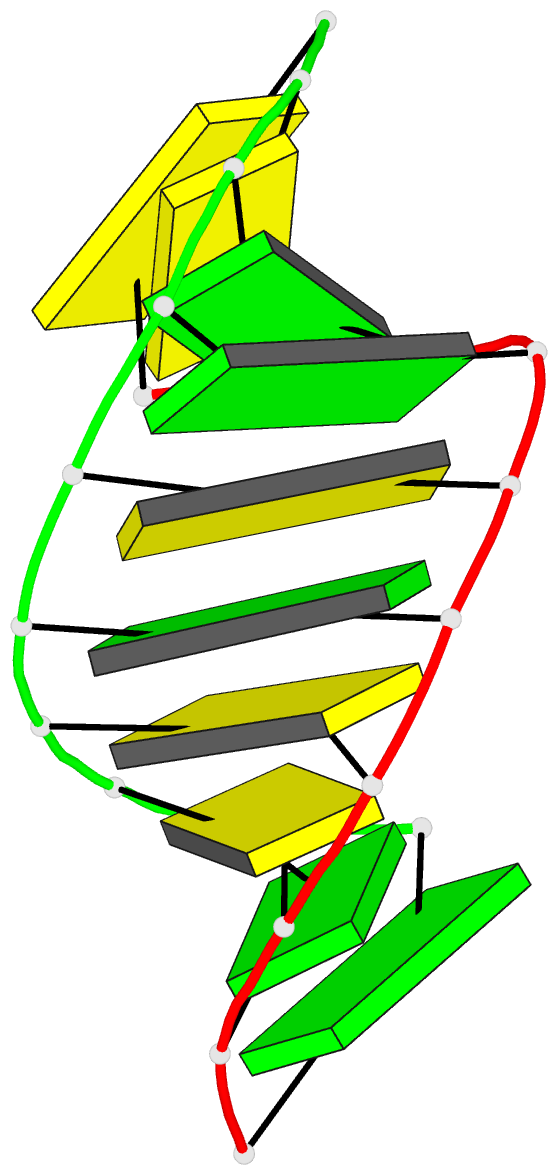
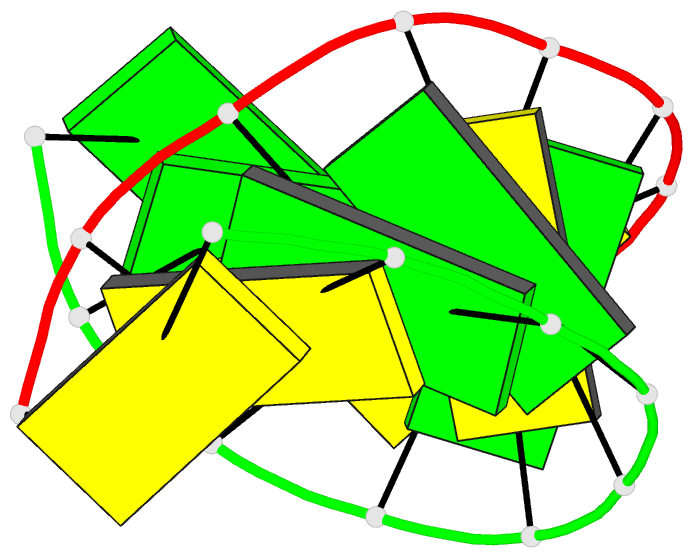
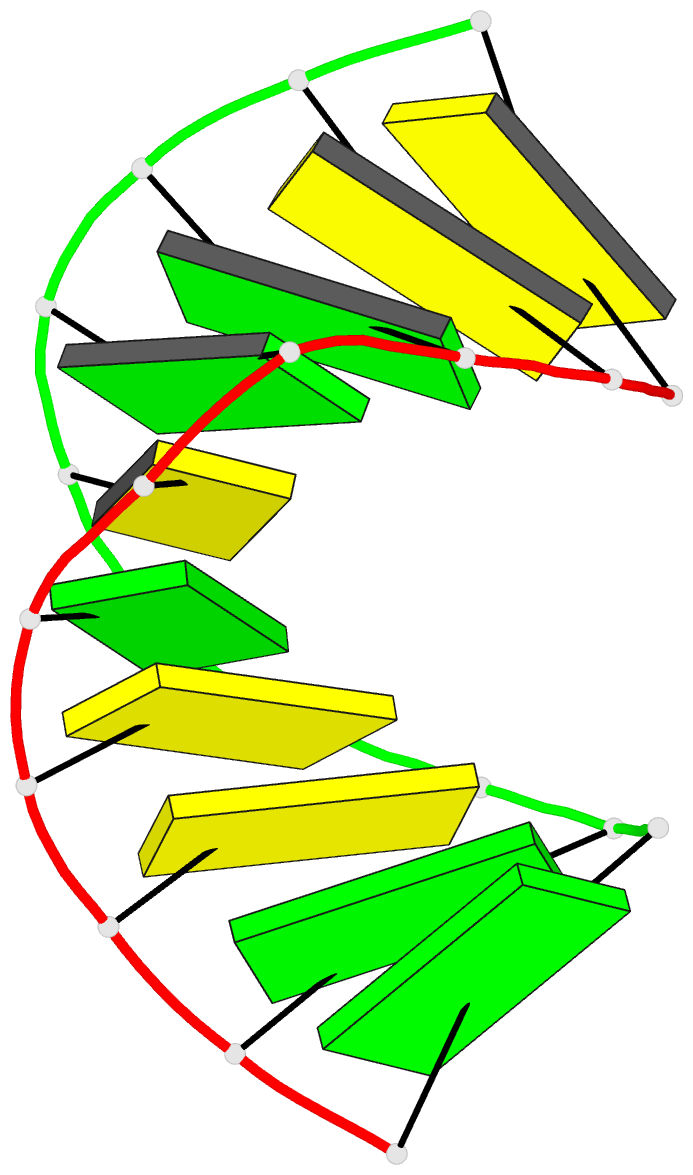
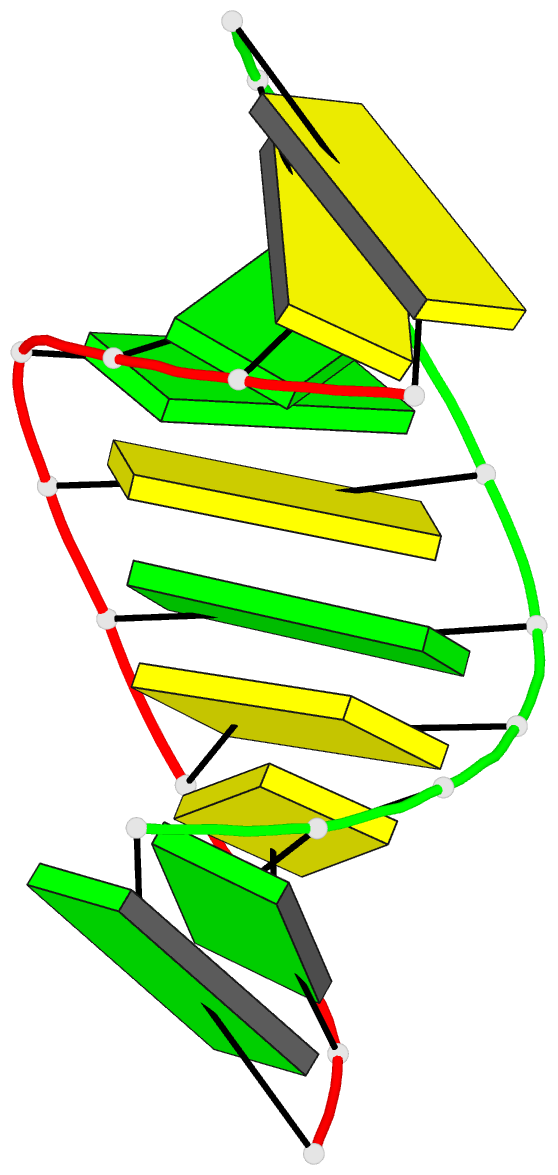
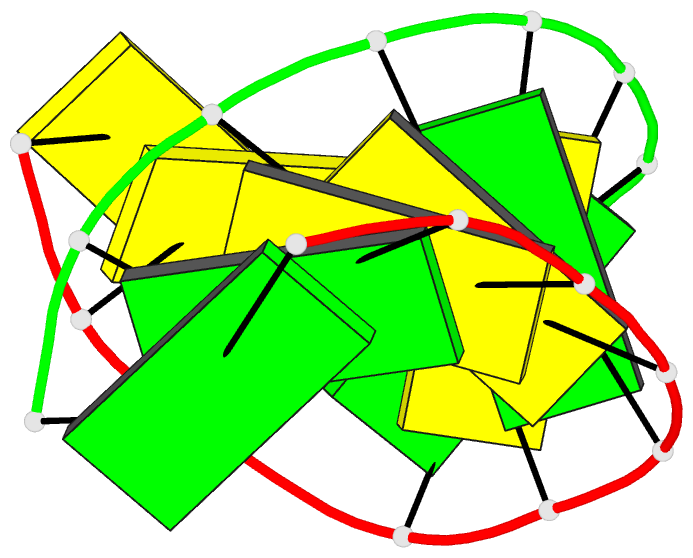
Summary information and primary citation
- PDB-id
-
161d;
SNAP-derived features in text and
JSON formats
- Class
- DNA-RNA hybrid
- Method
- X-ray (1.9 Å)
- Summary
- A single 2'-hydroxyl group converts b-DNA to a-DNA:
crystal structure of the DNA-RNA chimeric decamer duplex
d(ccggc)r(g)d(ccgg) with a novel intermolecular g.c
base-paired quadruplet
- Reference
-
Ban C, Ramakrishnan B, Sundaralingam M (1994): "A single
2'-hydroxyl group converts B-DNA to A-DNA. Crystal
structure of the DNA-RNA chimeric decamer duplex
d(CCGGC)r(G)d(CCGG) with a novel intermolecular G-C
base-paired quadruplet." J.Mol.Biol.,
236, 275-285. doi: 10.1006/jmbi.1994.1134.
- Abstract
- We have found that the introduction of a single
2'-hydroxyl group on the sugar-phosphate backbone of the
B-DNA decamer d(CCGGCGCCGG) transforms it to A-DNA. Thus,
for the first time the X-ray structures of the same
sequence have been observed in both the A and B-DNA
conformations, permitting a comparison. Crystals of the
DNA-RNA chimeric decamer d(CCGGC)r(G)d(CCGG) belong to the
orthorhombic space group P2(1)2(1)2(1) with unit cell
dimensions a = 25.63 A, b = 45.24 A and c = 47.99 A, and
one decamer duplex in the asymmetric unit. The structure
was solved by a rigid body search using the coordinates of
the isomorphous structure d(CCCGGCCGGG) and refined to an R
value of 0.136 using 2753 unique reflections at 1.9 A
resolution. The final model contains 406 nucleotide atoms
and 61 water molecules. The chimeric duplex exhibits
typical A-DNA geometry, with all the sugars in the
C(3')-endo puckering and the base-pairs inclined and
displaced from the helix axis. The 2'-hydroxyl groups on
rG6 and rG16 protrude into the minor groove surface and
form different types of hydrogen bonds; that on strand 1
forms an intermolecular hydrogen bond with the furanose
ring O(4') of a symmetry-related C1 residue, while that on
strand 2 is involved in two water bridges. Crystal packing
forces the G4-G17 base-pair in the top half of the duplex
to slide significantly into the minor groove compared to
the corresponding G7-G14 base-pair in the bottom half,
resulting in these base-pairs exhibiting different base
stacking and intermolecular interactions. The base G4 of
the G4-G17 base-pair forms an unorthodox base "triple",
G4*(G10-C11), hydrogen-bonding through its minor groove
sites N(2) and N(3) to the minor groove atoms N(2) and O(2)
of both bases of the G10-C11 base-pair of a
symmetry-related molecule. The base G10 of this triple in
turn forms a second similar unorthodox base triple,
G10*(G3*C18), with the adjacent base-pair G3-C18 of the
duplex, thus G10 is involved in a double triple. On the
other hand, in the bottom half of the duplex, the C7-G14
base-pair is involved only in a single similar unorthodox
base triple with G20, (C7-G14)*G20, while the adjacent
base-pair rG6-C15 is involved in a novel quadruple with
C1-G20, (rG6-C15) *(C1-G20), where the latter base-pairs
are hydrogen-bonded to each other via the minor groove
sites G(N(2))...C(O(2)).(ABSTRACT TRUNCATED AT 400
WORDS)