Summary information and primary citation
- PDB-id
-
108d;
SNAP-derived features in text and
JSON formats
- Class
- DNA
- Method
- NMR
- Summary
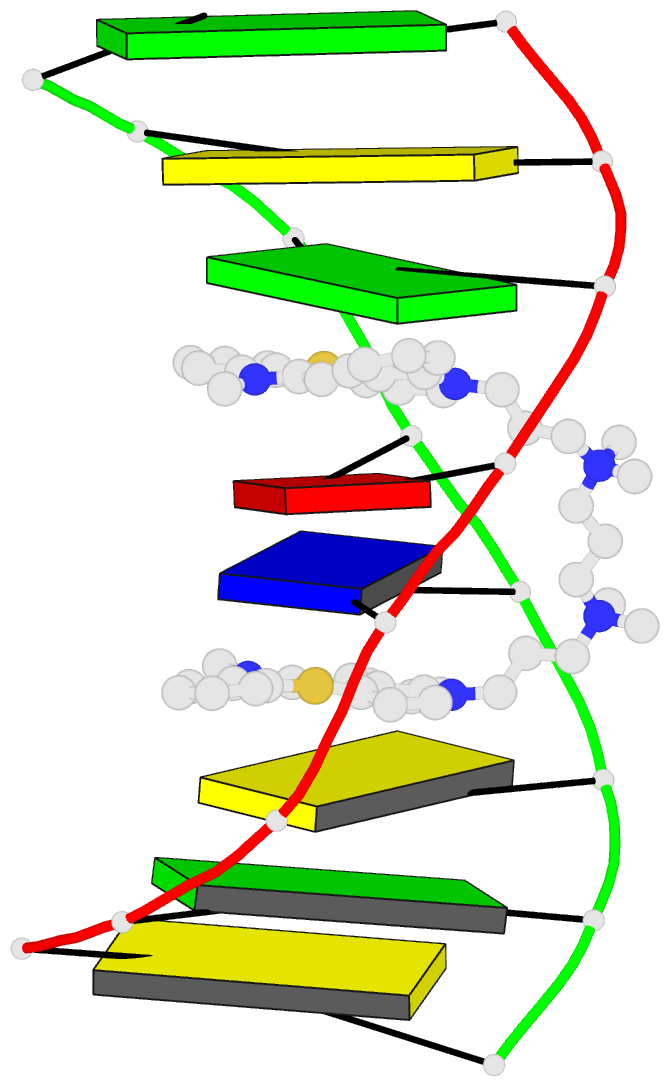
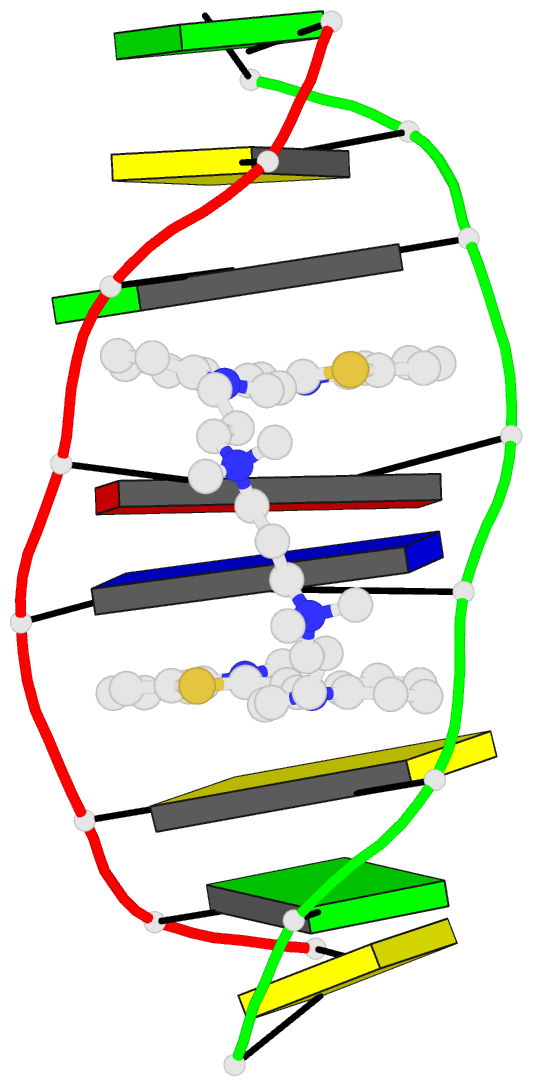
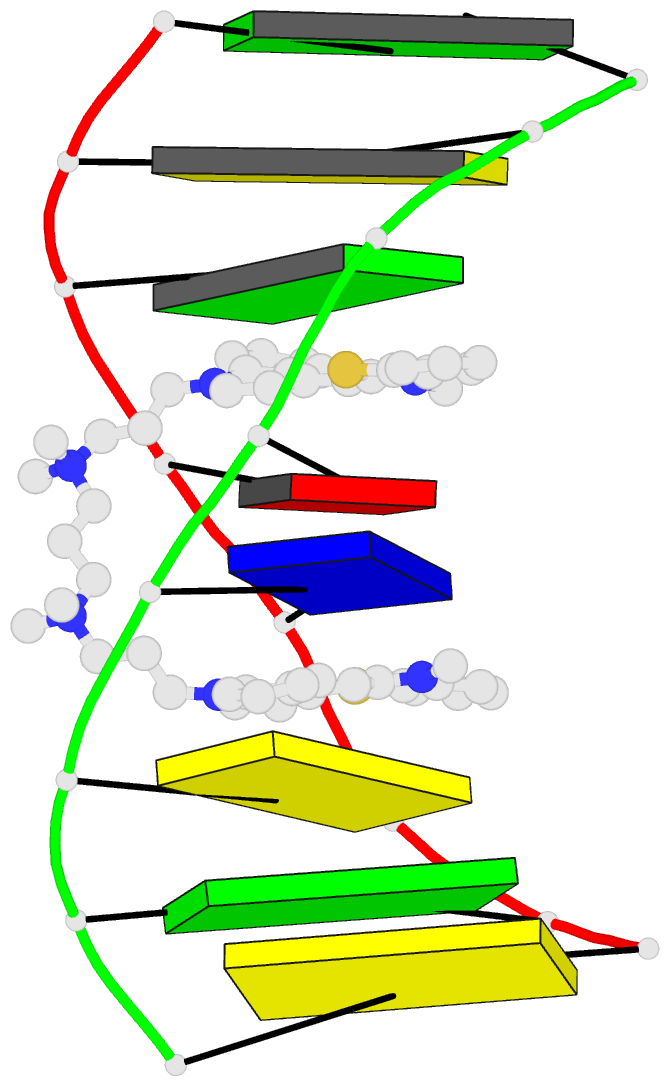
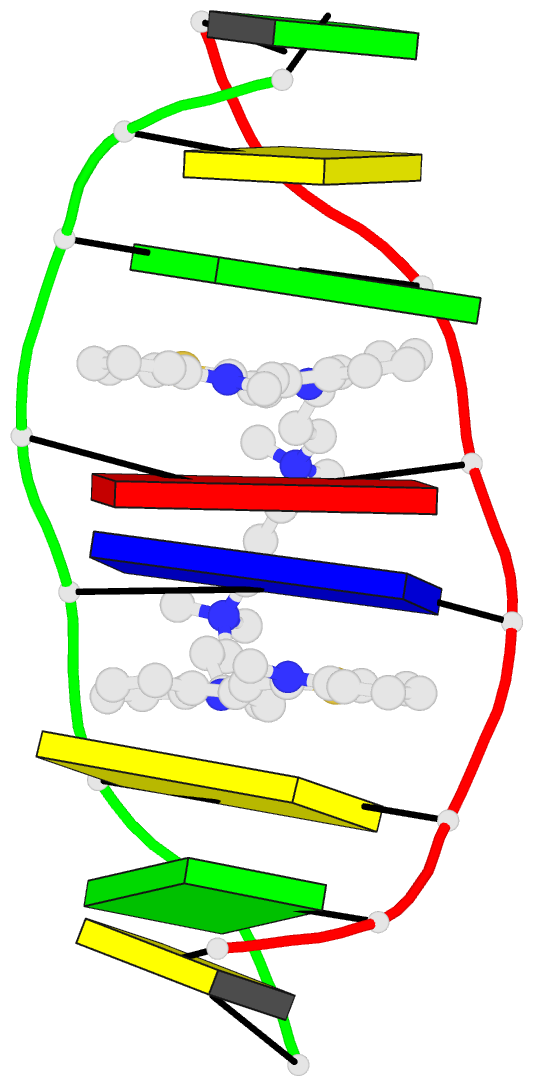
- The solution structure of a DNA complex with the
fluorescent bis intercalator toto determined by NMR
spectroscopy
- Reference
-
Spielmann HP, Wemmer DE, Jacobsen JP (1995): "Solution
structure of a DNA complex with the fluorescent
bis-intercalator TOTO determined by NMR
spectroscopy." Biochemistry,
34, 8542-8553. doi: 10.1021/bi00027a004.
- Abstract
- We have used two-dimensional 1H NMR spectroscopy to
determine the solution structure of the DNA oligonucleotide
d(5'-CGCTAGCG-3')2 complexed with the bis-intercalating dye
1,1'-(4,4,8,8-tetramethyl-4,8-diazaundecamethylene)bis[4-(3-methyl
-2,3- dihydrobenzo-1,3-thiazolyl-2-methylidene)qui
nolinium] tetraiodide (TOTO). The determination of the
structure was based on total relaxation matrix analysis of
the NOESY cross-peak intensities using the program
MARDIGRAS. Improved procedures to consider the experimental
"noise" in NOESY spectra during these calculations have
been employed. The NOE-derived distance restraints were
applied in restrained molecular dynamics calculations.
Twenty final structures each were generated for the TOTO
complex from both A-form and B-form dsDNA starting
structures. The root-mean-square (rms) deviation of the
coordinates for the 40 structures of the complex was 1.45
A. The local DNA structure is distorted in the complex. The
helix is unwound by 60 degrees and has an overall helical
repeat of 12 base pairs, caused by bis-intercalation of
TOTO. The poly(propylenamine) linker chain is located in
the minor groove of dsDNA. Calculations indicate that the
benzothiazole ring system is twisted relative to the
quinoline in the uncomplexed TOTO molecule. The site
selectivity of TOTO for the CTAG-CTAG site is explained by
its ability to adapt to the base pair propeller twist of
dsDNA to optimize stacking and the hydrophobic interaction
between the thymidine methyl group and the benzothiazole
ring. There is a 3000-fold fluorescence enhancement upon
binding of TOTO to dsDNA. Rotation about the cyanine
methine bonds is possible in free TOTO, allowing relaxation
nonradiatively. When bound to dsDNA, the benzothiazole ring
and the quinolinium ring are clamped by the nucleobases
preventing this rotation, and the chromophore loses
excitation energy by fluorescence instead.